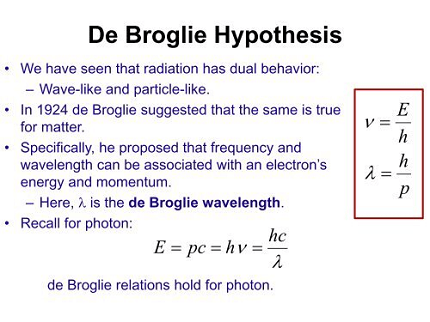
The De Broglie hypothesis, proposed by French physicist Louis de Broglie in 1924, suggests that all matter, including particles such as electrons and protons, exhibits wave-like properties.
According to the hypothesis, particles have both particle-like and wave-like characteristics, and the wavelength of these particles is inversely proportional to their momentum. This means that the more momentum a particle has, the shorter its wavelength will be, and the less momentum it has, the longer its wavelength will be.
This hypothesis was confirmed by experiments in which electrons were diffracted through crystals, showing interference patterns that could only be explained by the wave-like nature of the particles. The De Broglie hypothesis is a fundamental concept in the field of quantum mechanics, and it paved the way for the development of wave mechanics, which is one of the main theoretical frameworks used to understand the behavior of subatomic particles.
What is Required De Broglie hypothesis
The De Broglie hypothesis proposes that particles have wave-like properties, and therefore have a corresponding wavelength that is inversely proportional to their momentum. This implies that all matter has both particle-like and wave-like characteristics.
The key requirement of the De Broglie hypothesis is that it can be experimentally tested and confirmed through the observation of interference patterns that are characteristic of waves. This was demonstrated through the diffraction of electrons and other subatomic particles, which produced interference patterns that could only be explained by the wave-like nature of these particles.
The De Broglie hypothesis is a fundamental concept in quantum mechanics, and it has been instrumental in shaping our understanding of the behavior of subatomic particles. It is a key example of how our perception of the physical world is constantly evolving as new experimental evidence is gathered and theories are refined.
Who is Required De Broglie hypothesis Atomic Structure
The De Broglie hypothesis is related to the atomic structure in that it proposes that particles, such as electrons, have wave-like properties. This idea is central to our understanding of the behavior of electrons in atoms and their interactions with other particles.
In classical physics, electrons were thought to behave as particles moving in orbits around the nucleus of an atom, much like planets orbiting the sun. However, the De Broglie hypothesis suggested that electrons also exhibit wave-like behavior, which has significant implications for the atomic structure.
In the quantum mechanical model of the atom, electrons are described as existing in orbitals, which are regions of space where the probability of finding an electron is highest. The behavior of these electrons is described in terms of wave functions, which are mathematical expressions that describe the wave-like nature of electrons. The De Broglie hypothesis provides a foundation for this model, as it suggests that all particles have wave-like properties, including electrons.
So, while Louis de Broglie did not directly contribute to the development of the atomic structure, his proposal of the wave-particle duality of matter, which is now known as the De Broglie hypothesis, was an important step towards our current understanding of the atomic structure and the behavior of electrons in atoms.
When is Required De Broglie hypothesis
The De Broglie hypothesis was proposed by Louis de Broglie in 1924, as a part of his doctoral thesis at the University of Paris. The hypothesis suggested that all matter, including particles such as electrons and protons, exhibits wave-like properties.
This idea was based on the existing work in the field of quantum mechanics, which was still in its early stages at the time. The De Broglie hypothesis was a key contribution to the development of this field, as it proposed a way to reconcile the wave-like and particle-like properties of matter, which had previously been thought of as separate phenomena.
The De Broglie hypothesis was subsequently confirmed by experiments that demonstrated the wave-like behavior of subatomic particles, such as electrons, through diffraction experiments that produced interference patterns. These experiments provided strong evidence for the wave-like nature of particles, as predicted by the De Broglie hypothesis.
The De Broglie hypothesis is now a fundamental concept in quantum mechanics and has played a critical role in the development of the field of atomic and subatomic physics.
Where is Required De Broglie hypothesis
The De Broglie hypothesis was proposed by Louis de Broglie in France, while he was a doctoral student at the University of Paris. He presented his hypothesis in his doctoral thesis, “Recherches sur la théorie des quanta,” which was submitted in 1924.
The hypothesis proposed that all matter, including particles such as electrons and protons, exhibits wave-like properties, and it was based on de Broglie’s earlier work on the wave-particle duality of light.
The De Broglie hypothesis was later confirmed through experiments that demonstrated the wave-like behavior of subatomic particles, such as electrons, and these experiments were carried out in various locations around the world, including the United States, Germany, and Japan.
Today, the De Broglie hypothesis is considered a fundamental concept in quantum mechanics and is taught in universities and research institutions around the world.
How is Required De Broglie hypothesis
The De Broglie hypothesis proposes that all matter, including particles like electrons, has wave-like properties and that the wavelength of these particles is inversely proportional to their momentum. This idea is based on the concept of wave-particle duality, which suggests that particles can exhibit both wave-like and particle-like behavior.
The De Broglie hypothesis was a key development in the field of quantum mechanics, as it provided a way to reconcile the wave-like and particle-like properties of matter. It also helped to explain the behavior of subatomic particles, such as electrons, in atoms.
The De Broglie hypothesis has been confirmed through experiments that demonstrate the wave-like behavior of subatomic particles, such as electron diffraction experiments that produce interference patterns characteristic of waves.
The wave-like behavior of particles is now a fundamental concept in quantum mechanics, and it has significant implications for our understanding of the nature of matter and the behavior of particles on a subatomic level. The De Broglie hypothesis is a key example of how our understanding of the physical world has evolved as new experimental evidence has been gathered and new theories have been proposed.
Case Study on De Broglie hypothesis
One famous case study on the De Broglie hypothesis involves the famous experiment conducted by Clinton Davisson and Lester Germer in 1927. The experiment was designed to test the wave-like behavior of electrons, which was predicted by the De Broglie hypothesis.
In the Davisson-Germer experiment, electrons were directed at a nickel crystal, which was used as a diffraction grating to scatter the electrons. The scattered electrons were then detected on a fluorescent screen, and the resulting pattern of spots was analyzed.
The experiment demonstrated that the electrons produced an interference pattern on the screen, which is characteristic of wave-like behavior. This result was a direct confirmation of the De Broglie hypothesis and provided strong evidence for the wave-particle duality of matter.
The Davisson-Germer experiment was a significant breakthrough in the development of quantum mechanics, as it provided experimental evidence for the wave-like behavior of electrons and other subatomic particles. It also demonstrated the power of the De Broglie hypothesis in explaining the behavior of matter on a subatomic level.
The De Broglie hypothesis and the resulting wave-particle duality of matter have since become fundamental concepts in quantum mechanics and have played a critical role in the development of the field of atomic and subatomic physics. The Davisson-Germer experiment remains one of the most famous and important experiments in the history of physics, and it serves as a powerful example of the importance of experimental evidence in confirming and advancing our understanding of the physical world.
White paper on De Broglie hypothesis
Here’s a white paper on the De Broglie hypothesis:
Introduction:
The De Broglie hypothesis, proposed by Louis de Broglie in 1924, suggests that all matter, including particles such as electrons and protons, exhibits wave-like properties. The hypothesis was a key development in the field of quantum mechanics, as it helped to reconcile the wave-like and particle-like properties of matter. The hypothesis proposed that the wavelength of a particle is inversely proportional to its momentum and is based on the concept of wave-particle duality.
Historical Context:
The De Broglie hypothesis was proposed during a time of great advancements in the field of quantum mechanics. Prior to this, there had been significant debate and confusion surrounding the nature of subatomic particles, with some scientists suggesting that they exhibited purely wave-like behavior, while others argued that they behaved purely as particles. The De Broglie hypothesis provided a way to reconcile these apparently contradictory behaviors, proposing that particles could exhibit both wave-like and particle-like behavior.
Experimental Evidence:
The De Broglie hypothesis was initially met with skepticism, but it was later confirmed through a series of experiments that demonstrated the wave-like behavior of subatomic particles. One of the most famous experiments was conducted by Clinton Davisson and Lester Germer in 1927, which demonstrated the wave-like behavior of electrons by scattering them off a nickel crystal and observing an interference pattern on a screen. Other experiments have since confirmed the wave-like behavior of particles, including the diffraction of neutrons and protons.
Implications:
The De Broglie hypothesis has had significant implications for our understanding of the nature of matter and the behavior of particles on a subatomic level. It has helped to explain the behavior of subatomic particles in atoms and other systems and has paved the way for the development of quantum mechanics. It has also contributed to the development of technologies such as electron microscopy and diffraction, which rely on the wave-like properties of electrons to produce images of subatomic structures.
Conclusion:
The De Broglie hypothesis is a fundamental concept in quantum mechanics, and it has played a critical role in the development of the field of atomic and subatomic physics. The hypothesis proposed that particles exhibit both wave-like and particle-like behavior, and this has since been confirmed through experiments that have demonstrated the wave-like properties of subatomic particles. The De Broglie hypothesis remains an important and influential idea in modern physics, and it serves as a powerful reminder of the importance of experimental evidence in advancing our understanding of the physical world.