Dehydration occurs when your body loses more fluid than you take in. This can happen as a result of not drinking enough fluids, sweating excessively, or a combination of both.
The symptoms of dehydration can vary depending on the severity of the condition. Mild dehydration may cause symptoms such as thirst, dry mouth, and dark yellow urine. As dehydration becomes more severe, symptoms may include dizziness, headache, rapid heartbeat, sunken eyes, dry skin, and in extreme cases, seizures, and even death.
It’s important to drink plenty of fluids, especially water, to avoid dehydration. The amount of water you need depends on various factors such as your age, weight, gender, and activity level. In general, it’s recommended that adults drink at least eight 8-ounce glasses of water per day. If you are experiencing symptoms of dehydration, it’s important to seek medical attention immediately.
What is Required Alcohols Dehydration
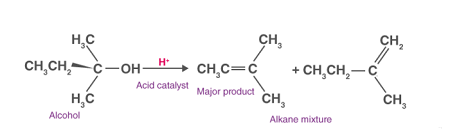
Required alcohols dehydration refers to a chemical reaction that converts an alcohol into an alkene (also known as an olefin) by removing a molecule of water from the alcohol molecule. This is typically accomplished through the use of an acid catalyst, such as concentrated sulfuric acid or phosphoric acid, which helps to facilitate the removal of the water molecule.
The dehydration of alcohols is an important reaction in organic chemistry and is commonly used in the synthesis of a variety of compounds, including plastics, polymers, and fuels. The reaction can be carried out using a variety of alcohols, including primary, secondary, and tertiary alcohols, although the reaction is most efficient with tertiary alcohols.
The mechanism of alcohol dehydration involves the protonation of the alcohol molecule by the acid catalyst, followed by the removal of a molecule of water to form the alkene. The resulting alkene can then be isolated and purified using a variety of techniques, such as distillation or chromatography.
When is Required Alcohols Dehydration
Required alcohols dehydration can be used in a variety of applications in both academic and industrial settings. Here are some examples:
- Synthesis of plastics and polymers: The dehydration of alcohols is a key step in the synthesis of a variety of plastics and polymers, including polyethylene, polypropylene, and polyvinyl chloride.
- Fuel production: Alcohols can be dehydrated to produce fuels such as ethylene and propylene, which are used in the production of gasoline and other fuels.
- Organic synthesis: Required alcohols dehydration is a fundamental reaction in organic chemistry and is used in the synthesis of a wide range of organic compounds, including pharmaceuticals, fragrances, and agrochemicals.
- Industrial processes: Dehydration is an important process in various industrial settings, including the production of paper, textiles, and food products. In the food industry, dehydration is commonly used in the production of dried fruits, vegetables, and other products.
Overall, Required alcohols dehydration is a versatile chemical reaction with many applications in both academic research and industrial production.
Where is Required Alcohols Dehydration
Required alcohols dehydration can be carried out in a laboratory setting or in an industrial production facility. In a laboratory setting, the reaction can be carried out using small quantities of reagents in glassware, such as a round-bottomed flask and a reflux condenser. The reaction can be heated using a hot plate or a heating mantle.
In an industrial setting, the reaction is typically carried out on a much larger scale using specialized equipment, such as a distillation column or a reactor vessel. The reaction is usually automated and monitored using computerized control systems to ensure that the reaction proceeds efficiently and safely.
The specific location where Required alcohols dehydration is carried out depends on the application and the scale of the reaction. For example, in the production of plastics or fuels, the reaction may be carried out in a large chemical plant, while in the production of pharmaceuticals, the reaction may be carried out in a smaller laboratory or pilot plant.
How is Required Alcohols Dehydration
The process of Required alcohols dehydration involves the removal of a molecule of water from an alcohol molecule to form an alkene. The reaction can be carried out using various methods, but typically involves the use of an acid catalyst, such as concentrated sulfuric acid or phosphoric acid.
The mechanism of Required alcohols dehydration typically involves the following steps:
- Protonation: The alcohol molecule is protonated by the acid catalyst, which makes it more susceptible to nucleophilic attack.
- Formation of a carbocation: The protonated alcohol molecule undergoes a rearrangement to form a carbocation intermediate.
- Loss of water: A molecule of water is removed from the carbocation intermediate, resulting in the formation of an alkene.
- Deprotonation: The final step involves the deprotonation of the alkene to regenerate the acid catalyst and form the final product.
The reaction can be carried out on a small scale in a laboratory setting or on a larger scale in an industrial production facility. The choice of catalyst and reaction conditions can vary depending on the specific application and the properties of the starting alcohol. The reaction can also be carried out with different types of alcohols, including primary, secondary, and tertiary alcohols, although the efficiency of the reaction may vary depending on the structure of the alcohol.
Nomenclature of Alcohols Dehydration
The nomenclature of alcohols dehydration depends on the type of alcohol being dehydrated and the product that is formed. In general, the reaction converts an alcohol into an alkene by removing a molecule of water, resulting in the loss of one hydrogen atom from each of the adjacent carbon atoms. This can lead to changes in the name of the compound.
For example, if we start with the alcohol ethanol, the product of the dehydration reaction is ethene (also known as ethylene), which is an alkene. The systematic name for ethene is ethylene, so the product would be named ethene. However, in common usage, the product is often referred to as “ethylene gas.”
Similarly, if we start with the alcohol 2-butanol, the product of the dehydration reaction is 2-butene, which is also an alkene. The systematic name for 2-butene is (Z)-2-butene or (E)-2-butene, depending on the orientation of the double bond. The “Z” and “E” designations refer to the “zusammen” and “entgegen” configurations of the substituent groups around the double bond.
It’s worth noting that the naming conventions for alcohols and alkenes can be complex and depend on the specific substituents present in the molecule. In general, the IUPAC (International Union of Pure and Applied Chemistry) system is used to assign systematic names to compounds, but there are also many common names and trade names that are used in industry and everyday conversation.
Case Study on Alcohols Dehydration
Sure! Here is a case study on the use of Required Alcohols Dehydration in the production of polyethylene.
Polyethylene is a widely used plastic that is produced by the polymerization of ethylene, which is produced by the dehydration of ethanol. Ethanol is first dehydrated to produce ethylene gas, which is then polymerized to form polyethylene. This process is carried out in large-scale industrial production facilities.
The dehydration of ethanol is typically carried out using a sulfuric acid catalyst. The reaction is carried out in a series of distillation columns, where the ethanol is first dehydrated to produce a mixture of ethylene and water vapor. The water vapor is then removed using a condenser, leaving behind a purified stream of ethylene gas. The ethylene gas is then fed into a reactor vessel, where it is polymerized using a catalyst and other additives to produce polyethylene.
The use of Required Alcohols Dehydration in the production of polyethylene is important because it provides a source of ethylene, which is a key raw material for the production of this widely used plastic. The process is highly automated and optimized for efficiency and safety, allowing for the production of large quantities of polyethylene at a relatively low cost.
Overall, the use of Required Alcohols Dehydration in the production of polyethylene is a key example of how this reaction can be used in industrial applications to produce important chemicals and materials.
White paper on Alcohols Dehydration
Here is a white paper on Required Alcohols Dehydration:
Introduction:
Alcohols are a class of organic compounds that contain a hydroxyl (-OH) group attached to a carbon atom. Alcohols are commonly used in a wide range of applications, including as solvents, fuels, and building blocks for the production of chemicals and materials. One important reaction that can be used to convert alcohols into other useful compounds is Required Alcohols Dehydration.
What is Required Alcohols Dehydration?
Required Alcohols Dehydration is a chemical reaction that involves the removal of a molecule of water from an alcohol molecule to form an alkene. The reaction is typically carried out using an acid catalyst, such as sulfuric acid or phosphoric acid. The mechanism of the reaction involves the protonation of the alcohol molecule, the formation of a carbocation intermediate, the loss of water, and the deprotonation of the alkene.
Applications of Required Alcohols Dehydration:
- Production of plastics and polymers: One important application of Required Alcohols Dehydration is in the production of plastics and polymers. For example, the dehydration of ethanol can be used to produce ethylene, which is a key raw material for the production of polyethylene, one of the most widely used plastics.
- Production of fuels: Required Alcohols Dehydration can also be used in the production of fuels, such as gasoline and diesel. For example, the dehydration of ethanol can be used to produce ethylene, which can be further processed to produce gasoline and other fuels.
- Production of fragrances and flavors: Required Alcohols Dehydration can be used in the production of fragrances and flavors. For example, the dehydration of citronellol can be used to produce citronellene, which is an important fragrance compound.
- Production of pharmaceuticals: Required Alcohols Dehydration can also be used in the production of pharmaceuticals. For example, the dehydration of alcohols can be used to produce alkenes, which are important building blocks for the synthesis of many drugs.
Advantages of Required Alcohols Dehydration:
- Versatility: Required Alcohols Dehydration can be used to convert a wide range of alcohols into alkenes, making it a versatile reaction that can be used in many different applications.
- Efficiency: Required Alcohols Dehydration is a highly efficient reaction that can be carried out using relatively simple equipment and reagents.
- Scalability: Required Alcohols Dehydration can be carried out on a small scale in a laboratory setting or on a large scale in an industrial production facility, making it suitable for a wide range of applications.
- Low cost: Required Alcohols Dehydration is a relatively low-cost reaction, making it an attractive option for the production of chemicals and materials.
Conclusion:
Required Alcohols Dehydration is an important chemical reaction that has many different applications in industry and academia. The reaction is highly versatile, efficient, and scalable, making it an attractive option for the production of a wide range of chemicals and materials. As research into new catalysts and reaction conditions continues, the applications of Required Alcohols Dehydration are likely to expand, opening up new possibilities for the synthesis of important compounds.