In many fields, including physics, chemistry, and engineering, the concept of an ideal situation or system is often used as a theoretical or idealized benchmark for comparison with real-world situations. However, in practice, it is rare to encounter a situation that perfectly conforms to the ideal model.
When a system deviates from the ideal model, this is referred to as a deviation from ideality. The degree of deviation can vary widely depending on the specific system or situation, but it can have significant implications for how the system behaves or performs.
For example, in physics, an ideal gas is a theoretical model that assumes that gas particles have no volume and do not interact with each other. However, in the real world, gases do have volume and can interact with each other, leading to deviations from the ideal gas law. In chemistry, the behavior of real solutions can deviate from the ideal behavior predicted by Raoult’s law, which assumes that the vapor pressure of a solution is proportional to the mole fraction of the solute.
In engineering, deviations from ideality can affect the performance of mechanical systems, such as engines or turbines. For example, friction and other sources of energy loss can cause a real-world engine to be less efficient than an idealized model.
In summary, deviations from ideality are a common occurrence in the real world, and understanding how and why a system deviates from the ideal model can be essential for designing and optimizing real-world systems.
What is Required Deviation from ideality Gases and Liquids
The required deviation from ideality for gases and liquids depends on the specific application or context. In some cases, a high degree of ideality may be desired or necessary, while in other cases, deviations from ideality may be acceptable or even desirable.
For example, in the design and operation of chemical processes, it is often necessary to take into account deviations from ideality in the behavior of gases and liquids in order to accurately predict the performance of the process. In these cases, accurate measurements of properties such as vapor pressure, solubility, and activity coefficients may be required.
On the other hand, in certain applications, such as the production of high-purity chemicals or pharmaceuticals, a high degree of ideality may be desired in order to ensure consistent product quality. In these cases, deviations from ideality can be minimized through careful selection of materials and operating conditions, as well as through the use of advanced separation and purification techniques.
In general, a thorough understanding of the properties and behavior of gases and liquids is essential for effectively designing and operating processes in a wide range of industries, from chemical and petrochemical production to food and beverage processing.
Who is Required Deviation from ideality Gases and Liquids
The concept of required deviation from ideality in gases and liquids is relevant to a wide range of fields, including physics, chemistry, chemical engineering, mechanical engineering, and materials science.
In physics and chemistry, understanding deviations from ideality in gases and liquids is important for predicting and explaining a variety of phenomena, such as phase transitions, solubility, and vapor-liquid equilibrium.
In chemical engineering, a thorough understanding of deviations from ideality is essential for designing and optimizing chemical processes, such as distillation, absorption, and extraction. Accurate predictions of properties such as vapor-liquid equilibrium, liquid-liquid equilibrium, and activity coefficients are critical for developing efficient and cost-effective processes.
In mechanical engineering, understanding deviations from ideality is important for designing and optimizing mechanical systems, such as engines, turbines, and compressors. Deviations from ideality in the behavior of gases and liquids can affect the performance and efficiency of these systems, and accurate predictions of properties such as viscosity, density, and thermal conductivity are critical for developing high-performance systems.
In materials science, understanding deviations from ideality is important for developing and characterizing materials with specific properties and behaviors. Deviations from ideality in the behavior of gases and liquids can affect the properties and behavior of materials, and accurate predictions of properties such as solubility and diffusivity are critical for developing materials with desired properties.
In summary, understanding and accounting for deviations from ideality in gases and liquids is essential for a wide range of scientific and engineering applications.
When is Required Deviation from ideality Gases and Liquids
The concept of required deviation from ideality in gases and liquids is relevant in a variety of situations and applications. Here are some examples:
- Chemical process design and optimization: In the design and optimization of chemical processes, it is often necessary to take into account deviations from ideality in the behavior of gases and liquids in order to accurately predict the performance of the process. Accurate measurements of properties such as vapor pressure, solubility, and activity coefficients may be required.
- Materials science: Understanding deviations from ideality in the behavior of gases and liquids is important for developing and characterizing materials with specific properties and behaviors. Deviations from ideality can affect the properties and behavior of materials, and accurate predictions of properties such as solubility and diffusivity are critical for developing materials with desired properties.
- Mechanical systems design: Deviations from ideality in the behavior of gases and liquids can affect the performance and efficiency of mechanical systems, such as engines, turbines, and compressors. Accurate predictions of properties such as viscosity, density, and thermal conductivity are critical for developing high-performance systems.
- Environmental science: Understanding deviations from ideality in the behavior of gases and liquids is important for predicting and mitigating environmental impacts, such as the behavior of pollutants in water or air.
- Pharmaceutical and food industry: In the production of high-purity chemicals or pharmaceuticals, a high degree of ideality may be desired in order to ensure consistent product quality. In these cases, deviations from ideality can be minimized through careful selection of materials and operating conditions, as well as through the use of advanced separation and purification techniques.
In general, the concept of required deviation from ideality in gases and liquids is relevant in any situation where accurate predictions or control of the behavior of these substances is important.
Where is Required Deviation from ideality Gases and Liquids
The concept of required deviation from ideality in gases and liquids is relevant in a wide range of fields and industries, and it can be found in many different locations and applications. Here are some examples:
- Chemical plants and refineries: The design and optimization of chemical processes often require accurate predictions of the behavior of gases and liquids, including deviations from ideality. Chemical plants and refineries are some of the most common locations where this concept is applied.
- Laboratories and research facilities: Researchers in fields such as chemistry, physics, and materials science may study the behavior of gases and liquids in a controlled environment, and may need to accurately measure deviations from ideality in order to understand and predict their properties and behaviors.
- Manufacturing facilities: The production of materials and products in industries such as pharmaceuticals, food and beverage, and semiconductors may require a high degree of ideality to ensure consistent product quality, and deviations from ideality may be closely monitored and controlled in these facilities.
- Environmental monitoring and remediation sites: The behavior of gases and liquids in the environment can have significant impacts on human health and the ecosystem, and deviations from ideality may be important factors to consider in monitoring and remediation efforts.
- Energy production facilities: The behavior of gases and liquids is critical to the performance and efficiency of energy production facilities, including power plants, refineries, and natural gas processing plants.
In general, required deviation from ideality in gases and liquids can be found in any location or application where accurate predictions or control of the behavior of these substances is important.
How is Required Deviation from ideality Gases and Liquids
The required deviation from ideality in gases and liquids can be calculated and predicted using a variety of methods and models. Here are some common approaches:
- Equations of State (EoS): An equation of state is a mathematical model that describes the thermodynamic behavior of gases and liquids. EoS models can be used to predict the required deviation from ideality, by incorporating parameters that account for intermolecular forces and non-ideality effects. Examples of EoS models include the Van der Waals, Peng-Robinson, and Soave-Redlich-Kwong equations of state.
- Activity Coefficient Models: Activity coefficient models are used to describe the deviations from ideality in liquid mixtures. These models include the Wilson, NRTL, and UNIQUAC models, which are commonly used in chemical process simulation software.
- Molecular Simulation: Molecular simulation techniques, such as Monte Carlo and molecular dynamics simulations, can be used to calculate the thermodynamic properties of gases and liquids at a molecular level. These methods can account for intermolecular forces and other non-ideal effects, and are particularly useful for studying complex mixtures and systems.
- Empirical correlations: Empirical correlations are mathematical relationships that have been developed through experimental data and observations. These correlations can be used to predict the required deviation from ideality in certain cases, such as the Antoine equation, which relates vapor pressure to temperature for pure liquids.
- Experimental measurements: Accurate measurements of thermodynamic properties, such as vapor-liquid equilibrium data, can be used to determine the required deviation from ideality in gases and liquids. Experimental measurements can be carried out using a variety of techniques, such as boiling point measurements, gas chromatography, and differential scanning calorimetry.
In summary, the required deviation from ideality in gases and liquids can be calculated and predicted using a range of methods and models, including equations of state, activity coefficient models, molecular simulation, empirical correlations, and experimental measurements. The choice of method depends on the specific application and the level of accuracy required.
Case Study on Deviation from ideality Gases and Liquids
One case study on deviation from ideality in gases and liquids involves the design and optimization of natural gas processing plants. Natural gas contains a mixture of hydrocarbon gases, including methane, ethane, propane, and butane, as well as impurities such as water vapor and sulfur compounds. The processing of natural gas involves separating these components and purifying them to meet market specifications.
The behavior of natural gas components is complex and non-ideal, due to intermolecular forces such as hydrogen bonding and dipole-dipole interactions. The deviation from ideality in natural gas can cause significant challenges in the design and operation of processing plants. For example, the non-ideality can lead to fouling of equipment, corrosion, and reduced efficiency.
To address these challenges, process engineers use a range of methods to predict and control the required deviation from ideality in natural gas. One approach is to use equations of state (EoS) models to calculate the thermodynamic properties of natural gas components. EoS models, such as the Peng-Robinson or Soave-Redlich-Kwong models, can account for non-ideal effects, such as intermolecular forces and vapor-liquid equilibria, to predict the behavior of natural gas components in processing plants.
Another approach is to use empirical correlations, such as the Lee-Kesler equation, to estimate the required deviation from ideality in natural gas. The Lee-Kesler equation relates the critical properties of natural gas components to their thermodynamic behavior, and can be used to predict phase equilibria, heat capacity, and other thermodynamic properties.
In addition to modeling and calculations, experimental measurements are also used to determine the required deviation from ideality in natural gas. For example, gas chromatography is commonly used to measure the composition of natural gas, while differential scanning calorimetry can be used to measure the heat capacity of natural gas components.
In summary, the required deviation from ideality in natural gas is a complex and important factor in the design and operation of processing plants. Process engineers use a range of methods, including EoS models, empirical correlations, and experimental measurements, to predict and control the non-ideality effects of natural gas components, and optimize the efficiency and performance of processing plants.
White paper on Deviation from ideality Gases and Liquids
Introduction
The behavior of gases and liquids can be described using the ideal gas and ideal solution models, respectively. However, in reality, gases and liquids deviate from ideality due to intermolecular forces and other non-ideal effects. These non-idealities can have significant implications for the design and operation of industrial processes, including natural gas processing, petroleum refining, and chemical manufacturing. In this white paper, we will explore the concept of deviation from ideality in gases and liquids, and its impact on industrial processes.
Deviation from Ideality in Gases
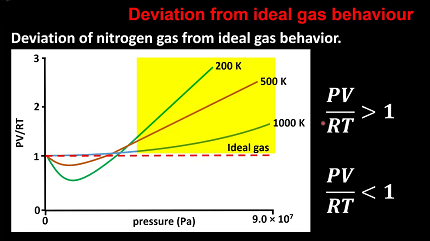
The ideal gas law, PV = nRT, is a fundamental relationship used to describe the behavior of gases. The ideal gas law assumes that gases are composed of particles with zero volume and no intermolecular forces, which is not the case in reality. Therefore, gases deviate from ideality due to the presence of intermolecular forces and finite particle volume.
The deviation from ideality in gases can be quantified using the compressibility factor, Z, which is defined as the ratio of the actual volume of a gas to the volume predicted by the ideal gas law. At low pressures and high temperatures, gases behave almost ideally, and Z is close to 1. At high pressures and low temperatures, gases deviate significantly from ideality, and Z is much greater than 1.
The deviation from ideality in gases can have significant implications for industrial processes. For example, natural gas processing plants rely on the separation and purification of hydrocarbon gases, including methane, ethane, propane, and butane. The behavior of these gases is complex and non-ideal, due to intermolecular forces such as hydrogen bonding and dipole-dipole interactions. The deviation from ideality in natural gas can cause significant challenges in the design and operation of processing plants, including fouling of equipment, corrosion, and reduced efficiency.
Deviation from Ideality in Liquids
Unlike gases, liquids do not follow a simple equation of state, such as the ideal gas law. The behavior of liquids is governed by a combination of intermolecular forces, such as van der Waals forces and hydrogen bonding, and entropy effects, such as the tendency of molecules to mix and form random arrangements.
The deviation from ideality in liquids can be quantified using activity coefficients, which are defined as the ratio of the actual activity of a component in a mixture to the activity predicted by Raoult’s law. Raoult’s law assumes that the vapor pressure of a liquid mixture is proportional to the mole fraction of each component in the mixture. However, in reality, intermolecular forces between different components can lead to deviations from Raoult’s law and non-ideal behavior.
The deviation from ideality in liquids can have significant implications for industrial processes. For example, in petroleum refining, the separation and purification of crude oil involves the use of distillation columns and other separation techniques. The behavior of crude oil components is complex and non-ideal, due to the presence of intermolecular forces and other non-ideal effects. The deviation from ideality in crude oil can cause significant challenges in the design and operation of distillation columns, including reduced separation efficiency and increased energy consumption.
Conclusion
In conclusion, deviation from ideality in gases and liquids is a complex and important factor in the design and operation of industrial processes. The non-ideal behavior of gases and liquids is due to intermolecular forces and other non-ideal effects, which can cause challenges in the design and operation of processing plants. Accurate modeling and prediction of non-ideal effects are crucial for optimizing the efficiency and performance of industrial processes, including natural gas processing, petroleum refining, and chemical manufacturing.
