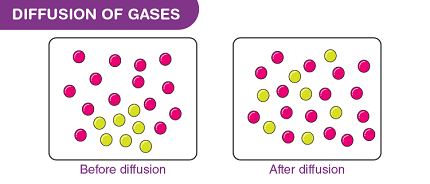
Diffusion is the process by which molecules or particles move from a region of high concentration to a region of low concentration. Diffusion of gases is an important physical process in various natural and industrial processes, including respiration, combustion, and chemical reactions.
In gases, diffusion occurs due to the random motion of gas molecules, also known as Brownian motion. The rate of diffusion is influenced by several factors, including temperature, pressure, molecular weight, and the size and shape of the molecules.
At a constant temperature, the rate of diffusion of gases is directly proportional to the partial pressure difference between the two regions. This is described by Graham’s law, which states that the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight.
Diffusion of gases can also occur through a membrane, which is known as membrane diffusion. This process is important in biological systems, where gases such as oxygen and carbon dioxide are exchanged across the respiratory membrane in the lungs.
Overall, diffusion of gases plays a critical role in many physical and biological processes, and understanding its principles is essential in a wide range of scientific fields.
Diffusion
Some particles are dissolved in a glass of water. At first, the particles are all near one top corner of the glass. If the particles randomly move around (“diffuse”) in the water, they eventually become distributed randomly and uniformly from an area of high concentration to an area of low, and organized (diffusion continues, but with no net flux).
Dissemination is the net development of anything (for instance, iotas, particles, atoms, energy) by and large from a district of higher focus to a locale of lower fixation. Dissemination is driven by a slope in Gibbs free energy or synthetic potential. It is feasible to diffuse “uphill” from a locale of lower fixation to a district of higher focus, as in spinodal deterioration. Dissemination is a stochastic interaction because of the intrinsic haphazardness of the diffusing substance and can be utilized to display some genuine stochastic situations. In this manner, dispersion and the relating numerical models has applications in a few fields, past physical science, for example, measurements, likelihood hypothesis, data hypothesis, brain organizations, money and showcasing and so on.
The idea of dispersion is broadly utilized in many fields, including physical science (molecule dissemination), science, science, humanism, financial aspects, measurements, information science and money (dispersion of individuals, thoughts, information and cost values). These fields utilize same or comparative numerical models, got from measurable material science, to show genuine stochastic frameworks. The focal thought of dissemination, in any case, is normal to these: a substance or assortment going through dispersion fans out from a point or area at which there is a higher centralization of that substance or assortment.
A slope is the adjustment of the worth of an amount, for instance, focus, strain, or temperature with the adjustment of another variable, normally distance. An adjustment of fixation over a distance is known as a focus slope, an adjustment of strain over a distance is known as a tension angle, and an adjustment of temperature over a distance is known as a temperature inclination.
The word dispersion gets from the Latin word, diffundere, and that signifies “to fan out”.
A distinctive element of dissemination is that it relies upon molecule irregular walk, and results in blending or mass vehicle without requiring coordinated mass movement. Mass movement, or mass stream, is the attribute of advection. The term convection is utilized to portray the mix of both vehicle peculiarities.
On the off chance that a dissemination cycle can be depicted by Fick’s regulations, it is known as a typical dispersion (or Fickian dissemination); In any case, it is called an odd dissemination (or non-Fickian dissemination).
While discussing the degree of dispersion, two length scales are utilized in two distinct situations:
- Brownian motion of an impulsive point source (for example, one single spray of perfume)—the square root of the mean squared displacement from this point. In Fickian diffusion, this is
- Constant concentration source in one dimension—the diffusion length. In Fickian diffusion, this is
Gaseous diffusion
Vaporous dissemination is an innovation that was utilized to create improved uranium by compelling vaporous uranium hexafluoride (UF6) through microporous films. This delivers a slight detachment (improvement factor 1.0043) between the particles containing uranium-235 (235U) and uranium-238 (238U). By utilization of a huge fountain of many stages, high partitions can be accomplished. It was the principal cycle to be fostered that was equipped for delivering improved uranium in mechanically helpful amounts, however is these days considered outdated, having been supplanted by the more-proficient gas axis process.
Vaporous dissemination was concocted by Francis Simon and Nicholas Kurti at the Clarendon Research facility in 1940, entrusted by the MAUD Board with tracking down a technique for isolating uranium-235 from uranium-238 to deliver a bomb for the English Cylinder Combinations project. The model vaporous dispersion hardware itself was produced by Metropolitan-Vickers (MetroVick) at Trafford Park, Manchester, at an expense of £150,000 for four units, for the M. S. Production line, Valley. This work was subsequently moved to the US when the Cylinder Compounds project became subsumed by the later Manhattan Project.
Rate of Diffusion
Dissemination is impacted by:
1.The fixation inclination – dissemination will be more noteworthy where slope is bigger
2. The temperatures – dissemination will happen quicker when temperatures are higher as there is more active energy
3. The surface region – dissemination will be more noteworthy where it’s more noteworthy
4. The dissemination distance – dispersion will be more noteworthy where there is a short dissemination distance
Molecular diffusion
Sub-atomic dissemination, frequently just called dispersion, is the warm movement of all (fluid or gas) particles at temperatures above outright zero. The pace of this development is an element of temperature, thickness of the liquid and the size (mass) of the particles. Dispersion makes sense of the net motion of particles from a locale of higher fixation to one of lower focus. When the focuses are equivalent the atoms keep on moving, however since there is no fixation inclination the course of sub-atomic dispersion has stopped and is rather administered by the course of self-dissemination, starting from the irregular movement of the particles. The consequence of dissemination is a slow blending of material to such an extent that the circulation of particles is uniform. Since the particles are still moving, however a harmony has been laid out, the consequence of sub-atomic dispersion is known as a “powerful balance”. In a stage with uniform temperature, missing outside net powers following up on the particles, the dissemination cycle will ultimately bring about complete blending.
Think about two frameworks; S1 and S2 at a similar temperature and equipped for trading particles. In the event that there is an adjustment of the expected energy of a framework; for instance μ1>μ2 (μ is Substance potential) an energy stream will happen from S1 to S2, on the grounds that nature generally favors low energy and most extreme entropy.
Sub-atomic dissemination is commonly portrayed numerically utilizing Fick’s laws of dispersion.
Mass diffusivity
Diffusivity, mass diffusivity or dispersion coefficient is generally composed as the proportionality consistent between the molar motion because of sub-atomic dissemination and the negative worth of the slope in the grouping of the species. All the more precisely, the dissemination coefficient times the nearby fixation is the proportionality consistent between the negative worth of the mole portion inclination and the molar transition. This differentiation is particularly critical in vaporous frameworks with solid temperature angles. Diffusivity gets its definition from Fick’s regulation and assumes a part in various different conditions of actual science.
The diffusivity is for the most part endorsed for a given sets of animal groups and pairwise for a multi-animal groups framework. The higher the diffusivity (of one substance as for another), the quicker they diffuse into one another. Regularly, a compound’s dispersion coefficient is ~10,000× as extraordinary in air as in water. Carbon dioxide in air has a dispersion coefficient of 16 mm2/s, and in water its dissemination coefficient is 0.0016 mm2/s.
Diffusivity has aspects of length2/time, or m2/s in SI units and cm2/s in CGS units.
Case Study on Diffusion of gases
One interesting case study on the diffusion of gases is the diffusion of carbon dioxide (CO2) into the atmosphere. CO2 is a greenhouse gas that plays a significant role in global warming and climate change.
CO2 is constantly being released into the atmosphere from natural sources such as volcanic eruptions and respiration of animals, as well as from human activities such as burning fossil fuels and deforestation. Once CO2 is released, it undergoes diffusion and mixing with the surrounding air.
The rate of diffusion of CO2 into the atmosphere is influenced by several factors, including the concentration gradient between the source and the surrounding air, wind speed, and atmospheric stability.
In recent years, the concentration of CO2 in the atmosphere has been increasing at an alarming rate, primarily due to human activities such as burning fossil fuels for energy production and transportation. This has led to concerns about the long-term effects of global warming and climate change.
Understanding the diffusion of CO2 into the atmosphere is important for predicting future changes in atmospheric composition and climate. It is also important for developing strategies to mitigate the effects of climate change, such as reducing greenhouse gas emissions and increasing the use of renewable energy sources.
White paper on Diffusion of gases
Introduction:
The diffusion of gases is a fundamental physical process that plays a critical role in many natural and industrial systems. In this white paper, we will discuss the principles of diffusion in gases, its importance in various scientific fields, and its applications in industry and technology.
Principles of Diffusion:
Diffusion is the process by which particles move from an area of high concentration to an area of low concentration. In gases, diffusion occurs due to the random motion of gas molecules. The rate of diffusion is influenced by several factors, including temperature, pressure, concentration gradient, and the properties of the gas molecules themselves (such as their size and shape).
One important concept in the diffusion of gases is Graham’s Law, which states that the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight. This means that lighter gases will diffuse more quickly than heavier gases.
Applications in Science and Industry:
The principles of diffusion in gases have important applications in a wide range of scientific fields, including physics, chemistry, biology, and environmental science. For example, in the human body, oxygen and carbon dioxide diffuse across the alveolar membrane in the lungs, and nutrients and waste products diffuse across cell membranes. In industry, diffusion is used in processes such as chromatography, gas separation, and wastewater treatment.
One important application of gas diffusion is in the production of semiconductors and other electronic devices. Diffusion is used to introduce impurities into the semiconductor material, which can alter its electrical properties and enable the production of complex circuits and devices.
Another important application of gas diffusion is in the development of fuel cells, which use the diffusion of gases such as hydrogen and oxygen to generate electricity. Fuel cells have the potential to be a clean and efficient source of energy, with applications in transportation, stationary power generation, and portable electronics.
Conclusion:
In conclusion, the diffusion of gases is a fundamental physical process that plays a critical role in many natural and industrial systems. Understanding the principles of gas diffusion is important for predicting the behavior of gases in various environments, as well as developing new technologies and applications. With ongoing research and innovation, the principles of gas diffusion are sure to continue to play a central role in science and industry in the years to come.