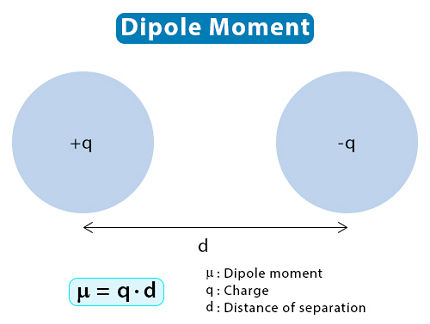
A dipole moment is a measure of the polarity of a molecule. It is defined as the product of the magnitude of the partial charges on two atoms in a molecule and the distance between them.
In other words, a dipole moment is the separation of positive and negative charges in a molecule, which results in a molecule having a partial positive and a partial negative end. This partial charge separation can occur due to differences in electronegativity between atoms in a molecule, resulting in the formation of polar covalent bonds.
Dipole moment is typically measured in debye units (D), which is equal to 3.336 × 10^-30 coulomb-meters (Cm). It is a vector quantity, meaning that it has both magnitude and direction. The direction of the dipole moment is from the negative end to the positive end of the molecule.
What is Required Dipole moment
A Required Dipole moment refers to the dipole moment that a molecule must possess in order to exhibit a certain physical or chemical behavior.
For example, in some chemical reactions, the reactants must have a certain dipole moment in order to undergo the reaction. Similarly, in some spectroscopic techniques, such as infrared spectroscopy, the dipole moment of a molecule is required to be non-zero in order for the technique to be effective.
The required dipole moment can also depend on the properties of the environment in which the molecule is located. For example, in solvents with high dielectric constants, polar molecules with higher dipole moments are more soluble and have higher boiling points than nonpolar molecules.
Therefore, the required dipole moment can vary depending on the specific application or behavior of interest.
When is Required Dipole moment
A Required Dipole moment is a concept in chemistry that refers to the dipole moment that a molecule must possess in order to exhibit a certain physical or chemical behavior or to be effective in a particular application.
For example, in some chemical reactions, the reactants must have a certain dipole moment in order to undergo the reaction. This is because the presence of a dipole moment can affect the electron distribution in a molecule, which can influence its reactivity.
In spectroscopic techniques, such as infrared spectroscopy, the dipole moment of a molecule is required to be non-zero in order for the technique to be effective. This is because the technique relies on the absorption of electromagnetic radiation by molecular vibrations, which are influenced by the dipole moment.
In addition, the required dipole moment can also depend on the properties of the environment in which the molecule is located. For example, in solvents with high dielectric constants, polar molecules with higher dipole moments are more soluble and have higher boiling points than nonpolar molecules.
Therefore, the concept of a required dipole moment is important in understanding the behavior and properties of molecules in various chemical and physical systems.
Production of Dipole moment
Dipole moment is not a substance that can be produced or synthesized. Instead, it is a property that arises from the arrangement of charges in a molecule. A molecule with a dipole moment has a separation of positive and negative charges, resulting in a net dipole moment.
The dipole moment of a molecule can be influenced by various factors, including the electronegativity of the atoms involved and the geometry of the molecule. For example, in a polar covalent bond, the electrons are shared unequally between the atoms, resulting in a separation of charge and the formation of a dipole moment. In a symmetric molecule, such as carbon dioxide (CO2), the dipole moments of the polar bonds cancel out, resulting in a nonpolar molecule with zero net dipole moment.
In summary, dipole moment is not produced or synthesized but is a property that arises from the arrangement of charges in a molecule.
Where is Required Dipole moment
A Required Dipole moment is a concept in chemistry and is not a physical location. It refers to the dipole moment that a molecule must possess in order to exhibit a certain behavior or property or to be effective in a particular application, as I explained in my previous responses.
The required dipole moment can depend on a variety of factors such as the properties of the environment, the type of chemical reaction or physical process, and the specific application or technique being used. It is a key concept in understanding the behavior of molecules and their interactions in various chemical and physical systems.
Therefore, the concept of a required dipole moment is applicable in many areas of chemistry, including organic chemistry, physical chemistry, and materials science, among others.
How is Required Dipole moment
The Required Dipole moment is a concept in chemistry that refers to the dipole moment that a molecule must possess in order to exhibit a certain physical or chemical behavior, or to be effective in a particular application. The required dipole moment can depend on a variety of factors, including the properties of the environment, the type of chemical reaction or physical process, and the specific application or technique being used.
The calculation of a required dipole moment involves analyzing the molecular structure and the electronegativity differences between the atoms in the molecule. The dipole moment is the product of the magnitude of the partial charges on two atoms in the molecule and the distance between them.
The required dipole moment can be determined by comparing the calculated dipole moment of a molecule with the minimum dipole moment required for a particular behavior or application. For example, in a chemical reaction that involves a polar reactant and a nonpolar reactant, the polar reactant must have a minimum dipole moment to undergo the reaction. Similarly, in a spectroscopic technique that relies on the absorption of electromagnetic radiation by molecular vibrations, the dipole moment of the molecule must be non-zero for the technique to be effective.
In addition to the calculation of the required dipole moment, experimental techniques such as NMR spectroscopy and X-ray crystallography can also be used to determine the dipole moment of a molecule and its behavior in various chemical and physical systems.
Case Study on Dipole moment
One example of the importance of dipole moment in chemistry is the study of the solubility of molecules in different solvents. The dipole moment of a molecule can affect its solubility in polar or nonpolar solvents.
For instance, consider the case of acetone (CH3COCH3), a polar molecule with a dipole moment of 2.91 Debye units. Acetone has a higher solubility in polar solvents like water or ethanol, which have high dielectric constants and can interact with the partial charges of the acetone molecule through dipole-dipole interactions. However, acetone has a lower solubility in nonpolar solvents like hexane, which have low dielectric constants and cannot interact with the polar molecule.
In contrast, consider the case of pentane (C5H12), a nonpolar molecule with a dipole moment of zero. Pentane has a higher solubility in nonpolar solvents like hexane, but a very low solubility in polar solvents like water or ethanol.
These examples demonstrate how the dipole moment of a molecule can affect its solubility in different solvents, which has important implications in various fields, such as drug discovery and environmental chemistry. For instance, the dipole moment of a drug molecule can affect its solubility in different biological fluids, which can impact its bioavailability and efficacy. Similarly, the dipole moment of pollutants in the environment can affect their solubility in water, soil, or air, which can affect their toxicity and persistence. Therefore, the study of dipole moment and its impact on chemical behavior is an important aspect of chemistry research.
White paper on Dipole moment
Dipole moment is a fundamental concept in chemistry that describes the separation of charge in a molecule, resulting in a polar molecule. The dipole moment is a vector quantity that represents the magnitude and direction of the separation of charge. In this white paper, we will explore the concept of dipole moment, its measurement, and its importance in various fields of chemistry.
Definition and Calculation of Dipole Moment
A dipole moment is defined as the product of the magnitude of the partial charges on two atoms in a molecule and the distance between them. The dipole moment is a vector quantity that is represented by an arrow pointing from the negative to the positive partial charge. The units of dipole moment are Debye (D), which is equal to 3.336 x 10^-30 coulomb-meters.
The dipole moment can be calculated using the following equation:
μ = Q x r
where μ is the dipole moment, Q is the magnitude of the partial charge, and r is the distance between the partial charges. The direction of the dipole moment is from the negative partial charge to the positive partial charge.
The dipole moment can also be calculated using molecular orbital theory, which describes the distribution of electrons in a molecule. The dipole moment can be derived from the molecular orbital coefficients and the distance between the atoms in the molecule.
Measurement of Dipole Moment
The dipole moment of a molecule can be measured experimentally using various techniques. One common method is through the measurement of dielectric constants, which are a measure of the ability of a substance to store electrical energy in an electric field. The dielectric constant of a substance can be related to its dipole moment through the following equation:
μ = (ε – ε_0) x V / E
where μ is the dipole moment, ε is the dielectric constant of the substance, ε_0 is the dielectric constant of vacuum, V is the volume of the substance, and E is the electric field.
Other methods for measuring dipole moment include NMR spectroscopy, X-ray crystallography, and infrared spectroscopy.
Importance of Dipole Moment
The dipole moment is an important concept in various fields of chemistry. For example, in organic chemistry, the dipole moment can affect the reactivity of a molecule in chemical reactions. Polar molecules with higher dipole moments are more reactive than nonpolar molecules because the presence of a dipole moment can influence the electron distribution in the molecule.
In physical chemistry, the dipole moment can affect the properties of a substance such as its boiling point, melting point, and solubility in different solvents. Polar molecules with higher dipole moments have higher boiling points and melting points than nonpolar molecules. They also have a higher solubility in polar solvents due to the ability of the solvent to interact with the partial charges of the molecule through dipole-dipole interactions.
In materials science, the dipole moment can affect the properties of materials such as their dielectric constants, ferroelectricity, and piezoelectricity. For example, the dipole moment in ferroelectric materials can be switched by an external electric field, resulting in the ability to store information.
Conclusion
The dipole moment is a fundamental concept in chemistry that describes the separation of charge in a molecule, resulting in a polar molecule. The dipole moment can be calculated using the partial charges and distance between the atoms in a molecule or derived from molecular orbital theory. The dipole moment can be measured experimentally using various techniques. The dipole moment is important in various fields of chemistry, including organic chemistry, physical chemistry, and materials science. Understanding the dipole moment and its impact on chemical behavior is an essential aspect of chemistry research.