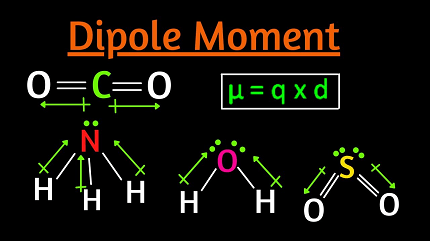
A dipole moment is a measure of the separation of electrical charge within a molecule or a polar covalent bond. It occurs when there is a separation of positive and negative charges within a molecule.
The magnitude of the dipole moment is represented by the product of the charge and the distance between the charges. The charge is expressed in terms of the partial charges of the atoms or groups of atoms within the molecule, and the distance between the charges is the distance between the centers of positive and negative charge.
Dipole moments are important in determining the physical and chemical properties of molecules. Polar molecules have a nonzero dipole moment, while nonpolar molecules have a dipole moment of zero. The dipole moment can affect the solubility, boiling and melting points, and reactivity of a molecule.
What is Required Alkenes and Alkynes Dipole moments
Alkenes and alkynes have a non-zero dipole moment if the molecule is asymmetrical, meaning that the bond dipoles do not cancel each other out.
In alkenes, the dipole moment is determined by the electronegativity difference between the carbon atoms and the attached atoms or groups of atoms. If the attached atoms or groups are different, then the molecule will have a dipole moment. For example, the dipole moment of ethene (C2H4) is 1.85 D, while the dipole moment of propene (C3H6) is 0.35 D.
In alkynes, the dipole moment is also determined by the electronegativity difference between the carbon atoms and the attached atoms or groups of atoms. If the attached atoms or groups are different, then the molecule will have a dipole moment. For example, the dipole moment of ethyne (C2H2) is 0.68 D, while the dipole moment of propyne (C3H4) is 0.36 D.
It’s important to note that if the molecule is symmetrical, meaning that the bond dipoles cancel each other out, then the molecule will have a dipole moment of zero. For example, the dipole moment of 1,3-butadiene (C4H6) is zero because the molecule is symmetrical.
When is Required Alkenes and Alkynes Dipole moments
The dipole moment of alkenes and alkynes occurs when the bond dipoles in the molecule do not cancel out. The bond dipole is a measure of the polarity of a chemical bond and arises due to differences in electronegativity between the two atoms forming the bond.
In alkenes and alkynes, the carbon-carbon double or triple bond is polarized due to the electronegativity difference between the carbon and the attached atoms or groups. The carbon atom in the double or triple bond has a partial positive charge, while the attached atom or group has a partial negative charge. As a result, a dipole moment is created, which can be measured experimentally.
However, the magnitude and direction of the dipole moment depend on the molecular geometry and the distribution of the partial charges within the molecule. If the molecule is symmetrical, the dipole moments of the individual bond dipoles may cancel each other out, resulting in a net dipole moment of zero.
Therefore, the dipole moment of an alkene or alkyne depends on the specific structure of the molecule, including the type and arrangement of the atoms or groups attached to the carbon-carbon double or triple bond.
Where is Required Alkenes and Alkynes Dipole moments
The dipole moment of alkenes and alkynes is a vector quantity that has both magnitude and direction. It points from the more electronegative atom or group of atoms towards the less electronegative one.
In alkenes, the dipole moment is directed along the line connecting the two carbon atoms, towards the more electronegative atom or group of atoms. This is because the double bond in an alkene is polarized due to the electronegativity difference between the carbon and the attached atoms or groups, creating a partial positive charge on the carbon atom and a partial negative charge on the attached atom or group.
In alkynes, the dipole moment is also directed along the line connecting the two carbon atoms, towards the more electronegative atom or group of atoms. This is because the triple bond in an alkyne is polarized due to the electronegativity difference between the carbon and the attached atoms or groups, creating a partial positive charge on the carbon atom and a partial negative charge on the attached atom or group.
The direction of the dipole moment is important in determining the physical and chemical properties of the molecule. For example, it can affect the solubility and reactivity of the molecule, as well as its interactions with electric fields and other polar molecules.
How is Required Alkenes and Alkynes Dipole moments
The dipole moment of alkenes and alkynes can be calculated or measured experimentally.
To calculate the dipole moment, the individual bond dipoles of the molecule must be considered. The bond dipole is a vector quantity that is proportional to the difference in electronegativity between the atoms forming the bond and the distance between them. The bond dipole is directed from the more electronegative atom towards the less electronegative atom.
The dipole moment of the molecule is then the vector sum of all the individual bond dipoles. This can be calculated using vector addition, taking into account both the magnitude and direction of each bond dipole.
Alternatively, the dipole moment of a molecule can be measured experimentally using techniques such as microwave spectroscopy or dielectric constant measurements. These methods measure the effect of the molecule’s dipole moment on its interaction with electric fields.
In either case, the dipole moment of alkenes and alkynes depends on the molecular geometry and the distribution of the partial charges within the molecule. It is also affected by the electronegativity difference between the atoms forming the double or triple bond and the type and arrangement of the atoms or groups attached to the carbon atoms in the bond.
Nomenclature of Alkenes and Alkynes Dipole moments
The nomenclature of alkenes and alkynes is based on the number and position of the double or triple bond in the carbon chain of the molecule. The main rules for naming these compounds are as follows:
- The parent chain of the molecule should be identified, which is the longest continuous chain of carbon atoms that contains the double or triple bond.
- The numbering of the parent chain should start from the end that is closest to the double or triple bond, and the double or triple bond should be given the lowest possible number.
- The suffix “-ene” is used for alkenes and “-yne” is used for alkynes.
- The position of the double or triple bond should be indicated by the number of the first carbon atom in the double or triple bond. The number is included before the suffix, separated by a hyphen.
- If there are multiple double or triple bonds in the molecule, the position and number of each bond should be indicated using prefixes such as “di-“, “tri-“, and so on.
For example, the compound with the formula C4H8 can be either 1-butene or 2-butene, depending on the position of the double bond in the parent chain. The compound with the formula C4H6 can be either 1-butyne or 2-butyne, again depending on the position of the triple bond in the parent chain.
The dipole moment of alkenes and alkynes can be indicated using the symbol “µ”, followed by the magnitude of the dipole moment in Debye units (D). For example, the dipole moment of ethene (C2H4) is 1.85 D, and the dipole moment of ethyne (C2H2) is 0.68 D. However, the dipole moment is not typically included in the systematic name of the molecule.
Case Study on Alkenes and Alkynes Dipole moments
Here is a case study on the dipole moments of alkenes and alkynes:
Case: A chemist is studying the solubility of alkenes and alkynes in water and other polar solvents. They want to know how the dipole moments of these molecules affect their interactions with water and other polar molecules.
Solution: The dipole moments of alkenes and alkynes can provide important information about their polarity and their ability to interact with polar solvents. For example, a higher dipole moment indicates a more polar molecule that can dissolve in polar solvents, while a lower dipole moment indicates a less polar molecule that is more likely to be soluble in nonpolar solvents.
To study the solubility of alkenes and alkynes in water and other polar solvents, the chemist can first determine the dipole moments of the molecules of interest. This can be done using experimental methods such as microwave spectroscopy or dielectric constant measurements, or through computational methods such as molecular modeling and quantum chemistry calculations.
Once the dipole moments are known, the chemist can compare them to the dipole moments of water and other polar solvents. Water has a dipole moment of 1.85 D, which is similar to the dipole moment of ethene (C2H4) at 1.85 D. This suggests that alkenes with similar dipole moments to ethene may be soluble in water. On the other hand, alkynes such as ethyne (C2H2) have lower dipole moments at 0.68 D, which may make them less soluble in water but more soluble in nonpolar solvents.
By considering the dipole moments of alkenes and alkynes, the chemist can predict their solubility in different types of solvents and design experiments to test their hypotheses. This information can also be used to understand the chemical reactivity of these compounds, as well as their potential uses in various applications such as materials science and pharmaceuticals.
White paper on Alkenes and Alkynes Dipole moments
Introduction:
Alkenes and alkynes are important classes of organic compounds that contain carbon-carbon double and triple bonds, respectively. These molecules have unique chemical and physical properties, including their dipole moments, which can influence their reactivity, solubility, and other properties. This white paper will provide an overview of the dipole moments of alkenes and alkynes, including their definition, measurement, and significance in chemistry and related fields.
What is a Dipole Moment?
A dipole moment is a measure of the separation of charge in a molecule. It is defined as the product of the magnitude of the partial charges on two atoms in a molecule and the distance between them. The dipole moment is expressed in units of Debye (D), which is equal to 3.33564 x 10^-30 coulomb meters. A molecule with a dipole moment has a permanent separation of charge, with one end being slightly positive and the other end being slightly negative.
Dipole Moments of Alkenes and Alkynes:
Alkenes and alkynes have dipole moments due to the polarity of their carbon-carbon double and triple bonds, respectively. The dipole moment of an alkene or alkyne depends on several factors, including the size and geometry of the molecule, the electronegativity of the atoms in the molecule, and the distribution of electrons around the double or triple bond.
In general, alkenes and alkynes with longer carbon chains tend to have higher dipole moments than those with shorter chains. This is because the longer chains have more electrons and greater electron density, which results in a larger separation of charge across the double or triple bond. Alkenes and alkynes with more highly electronegative atoms, such as oxygen or nitrogen, also tend to have higher dipole moments due to the stronger attraction between the atoms and their partial charges.
Measurement of Dipole Moments:
Dipole moments can be measured experimentally using various techniques, including microwave spectroscopy, dielectric constant measurements, and NMR spectroscopy. In microwave spectroscopy, the molecule is exposed to microwave radiation, and the dipole moment is determined from the frequency at which the molecule absorbs energy. In dielectric constant measurements, the dipole moment is calculated based on the ability of the molecule to align with an electric field. In NMR spectroscopy, the dipole moment is inferred from the chemical shift of the molecule in a magnetic field.
Significance of Dipole Moments:
The dipole moment of a molecule can provide important information about its reactivity, solubility, and other properties. For example, a higher dipole moment generally indicates a more polar molecule that can dissolve in polar solvents, while a lower dipole moment indicates a less polar molecule that is more likely to be soluble in nonpolar solvents. The dipole moment can also influence the chemical reactivity of the molecule, as it can affect the distribution of electrons and the stability of intermediates formed during reactions.
Conclusion:
The dipole moments of alkenes and alkynes are important properties that can provide insights into their chemical and physical properties. These dipole moments can be measured experimentally and used to predict the solubility, reactivity, and other properties of these compounds. Understanding the dipole moments of alkenes and alkynes can be useful in a variety of fields, including organic chemistry, materials science, and pharmaceuticals.
