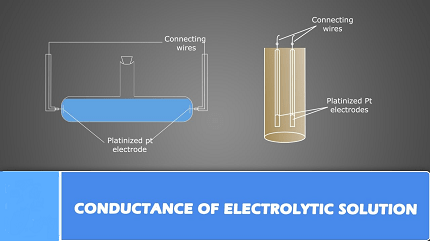
Electrolytic conductance refers to the ability of an electrolyte solution to conduct electric current. Electrolytes are substances that dissociate into ions when they dissolve in water, and these ions can move freely through the solution and carry electric charge.
The conductance of an electrolyte solution depends on several factors, including the concentration of ions in the solution, the size and charge of the ions, the temperature, and the presence of any other dissolved substances that may interact with the ions.
The unit of electrolytic conductance is Siemens per meter (S/m), which measures the conductivity of the solution per unit length. The reciprocal of electrolytic conductance is called electrolytic resistance, and it is measured in ohms (Ω).
What is Required Electrolytic conductance
Required electrolytic conductance refers to the minimum level of electrolytic conductance that is necessary for a particular application or process to function properly. The required conductance can vary depending on the specific application, but generally, a higher level of conductance is required for more demanding applications.
For example, in electroplating, a high level of electrolytic conductance is required to ensure a smooth and uniform coating on the surface of the metal being plated. If the conductance is too low, the plating may be uneven or incomplete.
Similarly, in batteries, a certain level of electrolytic conductance is required to allow for the efficient transfer of ions between the electrodes, which is necessary for the battery to produce and deliver electrical energy.
In general, the required level of electrolytic conductance will depend on the specific application and the performance requirements of the system.
When is Required Electrolytic conductance
The required electrolytic conductance will depend on factors such as the type of electrolyte being used, the concentration of the electrolyte, the temperature, and the electrical properties of the system. Once these factors are considered, the required conductance can be calculated or measured to ensure that the system or process will operate correctly and meet its performance requirements.
The required electrolytic conductance may also be periodically evaluated and adjusted during operation to ensure that the system continues to operate at optimal performance.
Where is Required Electrolytic conductance
“Required electrolytic conductance” is a specification or requirement that is relevant to any application or process that involves the use of an electrolyte solution. This can include a wide range of industries and applications, such as electroplating, battery manufacturing, chemical synthesis, and more.
The specific location of where the required electrolytic conductance is relevant will depend on the particular application or process. For example, in electroplating, the required conductance will be relevant at the plating tank where the electrolyte solution is contained, while in a battery, the required conductance will be relevant at the electrode surfaces where the electrolyte is in contact with the electrodes.
In general, the required electrolytic conductance is a specification that must be considered wherever an electrolyte solution is used, and it will be important to ensure that the appropriate level of conductance is maintained throughout the process or application.
How is Required Electrolytic conductance
“Required electrolytic conductance” is determined based on the performance requirements of the system or process that uses an electrolyte solution. The specific method for determining the required conductance will depend on the particular application, but in general, it involves considering factors such as:
- The type of electrolyte being used
- The concentration of the electrolyte
- The temperature of the solution
- The electrical properties of the system
Once these factors are considered, the required electrolytic conductance can be calculated or measured. The most common method for measuring electrolytic conductance is by using a conductivity meter, which measures the ability of the solution to conduct electrical current.
In some cases, the required electrolytic conductance may be established by industry standards or regulations that specify the minimum level of conductance that is necessary for a particular application. In other cases, it may be established through experimentation or testing to determine the optimal level of conductance for a given system.
Once the required electrolytic conductance has been determined, it is important to ensure that it is maintained throughout the process or application to ensure optimal performance. This may involve monitoring the conductivity of the solution and making adjustments as necessary to maintain the required level of conductance.
Nomenclature of Electrolytic conductance
The nomenclature of electrolytic conductance includes several terms that are commonly used in the study of electrolyte solutions and their conductivity. Some of the most important terms include:
- Electrolyte: A substance that dissociates into ions when it dissolves in a solvent such as water.
- Ion: An atom or molecule that has an electric charge due to the gain or loss of one or more electrons.
- Cation: A positively charged ion that is attracted to the negative electrode in an electrolytic cell.
- Anion: A negatively charged ion that is attracted to the positive electrode in an electrolytic cell.
- Conductance: The ability of a substance to conduct electrical current, measured in Siemens (S).
- Resistance: The opposition of a substance to the flow of electrical current, measured in ohms (Ω).
- Specific conductance: The conductance of a substance normalized to a standard concentration and cell geometry, measured in Siemens per centimeter (S/cm).
- Molar conductance: The conductance of a solution containing one mole of solute per liter of solution, measured in Siemens per meter per mole (S m^2 mol^-1).
- Equivalent conductance: The conductance of a solution containing one equivalent of solute per liter of solution, measured in Siemens per meter per equivalent (S m^2 eq^-1).
These terms are important for describing and understanding the behavior of electrolyte solutions and their conductivity.
Case Study on Electrolytic conductance
Here is a hypothetical case study on the importance of electrolytic conductance in battery manufacturing:
ABC Battery Company produces lithium-ion batteries for use in electric vehicles. The company has been experiencing issues with the performance and lifespan of their batteries, and they suspect that electrolyte composition may be a contributing factor.
To investigate this, the company decides to measure the electrolytic conductance of their electrolyte solution and compare it to industry standards. They use a conductivity meter to measure the specific conductance of the solution at different temperatures and concentrations.
The results of their tests show that the electrolytic conductance of their solution is significantly lower than the industry standard for lithium-ion batteries. This means that the solution is not able to efficiently conduct electrical current, which can result in lower battery performance and shorter lifespan.
To address this issue, the company decides to adjust the concentration of their electrolyte solution and measure the conductance again. After several rounds of testing, they find that increasing the concentration of the electrolyte solution to a certain level significantly increases its conductance, and they are able to meet the industry standard.
As a result of this investigation, the company is able to improve the performance and lifespan of their batteries by optimizing their electrolyte composition to ensure optimal electrolytic conductance. They are also able to increase customer satisfaction and maintain their position as a leading producer of lithium-ion batteries for electric vehicles.
White paper on Electrolytic conductance
Introduction:
Electrolytic conductance is the ability of an electrolyte solution to conduct electrical current. Electrolyte solutions are made up of ions that can carry an electrical charge, and the movement of these ions in solution allows the solution to conduct electricity. Electrolytic conductance is an important property of electrolyte solutions and is relevant to a wide range of industries and applications, including battery manufacturing, electroplating, and chemical synthesis.
This white paper will provide an overview of electrolytic conductance, including its measurement, factors affecting its value, and its importance in various applications.
Measurement of Electrolytic Conductance:
The conductivity of an electrolyte solution is typically measured using a conductivity meter, which measures the ability of the solution to conduct an electrical current. The specific conductance of the solution is the conductance measured between two electrodes in a cell with a known geometry and a known distance between the electrodes. The specific conductance is typically reported in Siemens per centimeter (S/cm).
Factors Affecting Electrolytic Conductance:
The value of electrolytic conductance can be affected by several factors, including the concentration of the electrolyte, the temperature of the solution, and the electrical properties of the system. In general, as the concentration of the electrolyte increases, the electrolytic conductance also increases. This is because a higher concentration of ions in the solution allows for more effective charge transport.
The temperature of the solution also affects electrolytic conductance. As the temperature increases, the kinetic energy of the ions increases, which promotes faster ion movement and higher conductance. However, at very high temperatures, electrolytic conductance may decrease due to other factors, such as increased ion-pairing.
The electrical properties of the system, including the type of ions in the solution and the nature of the electrodes, can also affect the electrolytic conductance. The presence of certain ions may promote ion-pairing or other interactions that reduce conductance, while the nature of the electrodes may affect the surface charge and affect the movement of ions in the solution.
Importance of Electrolytic Conductance:
Electrolytic conductance is an important property in many applications, including battery manufacturing, electroplating, and chemical synthesis. In battery manufacturing, the conductance of the electrolyte solution can affect the performance and lifespan of the battery. A higher conductance can promote more efficient charge transport and lead to higher battery performance.
In electroplating, electrolytic conductance is important for ensuring uniform deposition of the plating material. A lower conductance can result in uneven deposition and poor plating quality.
In chemical synthesis, electrolytic conductance is important for controlling reaction rates and yields. A higher conductance can promote faster reaction rates and higher yields, while a lower conductance may result in slower reactions and lower yields.
Conclusion:
Electrolytic conductance is an important property of electrolyte solutions that can affect the performance of a wide range of applications. Its value is affected by several factors, including the concentration of the electrolyte, the temperature of the solution, and the electrical properties of the system. Measuring and optimizing electrolytic conductance can lead to improved performance and quality in various applications, making it a key consideration in many industries.
