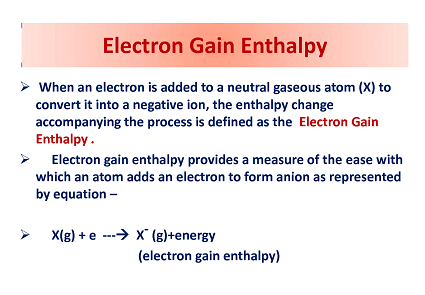
Electron gain enthalpy, also known as electron affinity, is a measure of the energy change that occurs when an atom or molecule gains an electron. It is defined as the enthalpy change that occurs when a neutral gaseous atom gains an extra electron to form a negatively charged ion.
The electron gain enthalpy is generally expressed in units of kilojoules per mole (kJ/mol). A negative value of electron gain enthalpy indicates that the process of gaining an electron is exothermic, meaning that energy is released. A positive value of electron gain enthalpy indicates that the process is endothermic, meaning that energy is required to add an electron.
The electron gain enthalpy of an element depends on factors such as the atomic size, electronic configuration, and the effective nuclear charge experienced by the atom. Elements with smaller atomic size and higher effective nuclear charge generally have a greater affinity for electrons and thus a more negative electron gain enthalpy. However, there are some exceptions to this trend, such as the noble gases, which have a very low electron affinity due to their stable electronic configuration.
What is Required Electron gain enthalpy
Required Electron gain enthalpy refers to the minimum amount of energy that is required to add an electron to an atom or molecule. This is different from the standard electron gain enthalpy, which is the energy change that occurs when an atom or molecule gains an electron under standard conditions.
The required electron gain enthalpy depends on the specific conditions of the system and may vary depending on factors such as the temperature, pressure, and the chemical environment of the atom or molecule. It is also influenced by the electronic structure and the nature of the atom or molecule itself.
In general, the required electron gain enthalpy is higher than the standard electron gain enthalpy, as the process of adding an electron becomes more difficult when the atom or molecule is already negatively charged or in a chemically complex environment.
When is Required Electron gain enthalpy
The Required Electron gain enthalpy is the energy required to add an electron to an atom or molecule under non-standard conditions, such as high temperatures or pressures. It can also refer to the energy required to add an electron to an atom or molecule in a particular chemical environment, such as in a solvent or in the presence of other ions or molecules.
The Required Electron gain enthalpy can be influenced by various factors, including the electronic structure of the atom or molecule, the size and shape of the atom or molecule, and the chemical environment in which the process takes place. For example, the presence of other ions or molecules can affect the electron affinity of an atom or molecule, making it more or less likely to accept an additional electron.
In general, the Required Electron gain enthalpy is higher than the standard electron gain enthalpy, since the process of adding an electron becomes more difficult under non-standard conditions or in complex chemical environments. However, the exact value of the Required Electron gain enthalpy will depend on the specific conditions of the system in question.
Where is Required Electron gain enthalpy
The Required Electron gain enthalpy is a term used in chemistry to describe the energy required to add an electron to an atom or molecule under non-standard conditions. It is a concept used to understand the behavior of atoms and molecules in different chemical environments, and is an important consideration in fields such as materials science, chemical engineering, and biochemistry.
The Required Electron gain enthalpy is a property of the atom or molecule, and its value depends on various factors such as the electronic structure, the size and shape of the atom or molecule, and the chemical environment in which the process takes place. It is typically measured in units of energy per mole, such as joules per mole (J/mol) or kilojoules per mole (kJ/mol).
The concept of Required Electron gain enthalpy is related to the standard electron gain enthalpy, which is the energy change that occurs when an atom or molecule gains an electron under standard conditions. However, the Required Electron gain enthalpy takes into account the influence of non-standard conditions or complex chemical environments, which can affect the electron affinity of the atom or molecule.
How is Required Electron gain enthalpy
The Required Electron gain enthalpy is the amount of energy required to add an electron to an atom or molecule under non-standard conditions, such as high temperatures or pressures, or in the presence of other ions or molecules. This energy can be calculated using various theoretical models, such as quantum mechanics or thermodynamics.
In general, the Required Electron gain enthalpy is higher than the standard electron gain enthalpy, since the process of adding an electron becomes more difficult under non-standard conditions or in complex chemical environments. The calculation of the Required Electron gain enthalpy takes into account the influence of these factors on the electron affinity of the atom or molecule.
The calculation of the Required Electron gain enthalpy involves determining the change in energy that occurs when an electron is added to the atom or molecule in question. This can be done using various methods, such as density functional theory (DFT), Hartree-Fock theory, or molecular orbital theory. The results of these calculations can then be used to understand the behavior of the atom or molecule in different chemical environments, and to design new materials or chemical processes.
Nomenclature of Electron gain enthalpy
The nomenclature of Electron gain enthalpy refers to the naming conventions and units used to describe this physical property in chemistry. The Electron gain enthalpy is a measure of the energy change that occurs when an atom or molecule gains an electron, and it is typically expressed in units of energy per mole, such as joules per mole (J/mol) or kilojoules per mole (kJ/mol).
The sign of the Electron gain enthalpy value indicates whether the process of adding an electron to the atom or molecule is exothermic (releasing energy) or endothermic (absorbing energy). A negative Electron gain enthalpy value indicates an exothermic process, while a positive value indicates an endothermic process.
There are different naming conventions used for Electron gain enthalpy depending on the context. In general, the term “Electron gain enthalpy” is commonly used in reference to the energy change that occurs when a neutral gaseous atom gains an electron to form a negatively charged ion. The term “Electron affinity” is also sometimes used interchangeably with Electron gain enthalpy, although this term can also refer to the energy change that occurs when an electron is added to a solid material.
It is important to note that the value of the Electron gain enthalpy can vary depending on the specific conditions of the system, such as temperature, pressure, and the chemical environment of the atom or molecule. Therefore, it is often necessary to specify the conditions under which the Electron gain enthalpy value was determined when reporting or discussing this property in scientific literature.
Case Study on Electron gain enthalpy
One example of a case study on Electron gain enthalpy involves the behavior of the halogens (Group 17 elements) in the periodic table. The Electron gain enthalpy of the halogens is an important factor that influences their reactivity and chemical behavior, as well as their ability to form covalent and ionic compounds with other elements.
The Electron gain enthalpy of the halogens increases from fluorine to iodine due to the increasing size of the atoms, which leads to a greater attraction between the positively charged nucleus and the incoming negatively charged electron. However, there is a notable exception for the Electron gain enthalpy of fluorine, which is lower than that of chlorine despite fluorine being smaller in size.
This anomaly can be explained by the relatively high electron density of the fluorine atom, which results in strong repulsive forces between the incoming electron and the existing electrons in the valence shell. As a result, it is energetically unfavorable for fluorine to accept an additional electron, leading to a lower Electron gain enthalpy value.
The behavior of the halogens can also be influenced by other factors such as the chemical environment in which the Electron gain enthalpy measurement is taken. For example, the presence of other ions or molecules can affect the Electron gain enthalpy by altering the electron affinity of the halogen atom.
Understanding the Electron gain enthalpy of the halogens is important in predicting their behavior in chemical reactions, such as their ability to form covalent bonds with other non-metallic elements or ionic bonds with metallic elements. It also has practical applications in industries such as materials science and chemical engineering, where the reactivity of halogens is important in the production of various chemical compounds and materials.
White paper on Electron gain enthalpy
Title: An Overview of Electron Gain Enthalpy in Chemistry
Abstract: Electron gain enthalpy is a fundamental property of atoms and molecules that is critical to understanding their behavior in chemical reactions. In this white paper, we provide an overview of Electron gain enthalpy, including its definition, significance, and applications in various areas of chemistry. We discuss the factors that influence Electron gain enthalpy, such as atomic size, electronic configuration, and chemical environment, and we examine the trends in Electron gain enthalpy across different groups of elements in the periodic table. Additionally, we highlight the practical applications of Electron gain enthalpy in materials science, chemical engineering, and other industries, and we discuss the challenges associated with measuring Electron gain enthalpy accurately. This paper aims to provide a comprehensive introduction to Electron gain enthalpy, with the goal of enhancing understanding of this important property among students, researchers, and professionals in the field of chemistry.
Introduction: Electron gain enthalpy is a measure of the energy change that occurs when an atom or molecule gains an electron. It is a fundamental property of atoms and molecules that plays a critical role in determining their chemical behavior. Understanding Electron gain enthalpy is essential to predicting the reactivity of atoms and molecules in chemical reactions, as well as in designing new materials and chemical processes.
Definition and Significance: The Electron gain enthalpy of an atom or molecule is the energy change that occurs when an electron is added to a neutral atom or molecule in the gaseous phase. It is typically expressed in units of energy per mole, such as joules per mole (J/mol) or kilojoules per mole (kJ/mol). The sign of the Electron gain enthalpy value indicates whether the process of adding an electron is exothermic (releasing energy) or endothermic (absorbing energy). A negative value of Electron gain enthalpy indicates an exothermic process, while a positive value indicates an endothermic process.
The Electron gain enthalpy is a crucial property in understanding the chemical behavior of atoms and molecules. It helps to explain why some atoms and molecules are more reactive than others, and why certain chemical reactions occur while others do not. It is also used to predict the ability of atoms and molecules to form covalent and ionic bonds with other elements.
Factors Affecting Electron Gain Enthalpy: Several factors can influence the value of the Electron gain enthalpy of an atom or molecule, including atomic size, electronic configuration, and chemical environment. Larger atoms generally have lower Electron gain enthalpy values than smaller atoms, as the incoming electron is farther from the positively charged nucleus and is therefore less strongly attracted. The electronic configuration of the atom or molecule also affects Electron gain enthalpy, as the addition of an electron may result in a filled or half-filled electron shell, which is energetically favorable. The chemical environment, such as the presence of other ions or molecules, can also influence the value of the Electron gain enthalpy by altering the electron affinity of the atom or molecule.
Trends in Electron Gain Enthalpy: The Electron gain enthalpy of atoms and molecules varies across different groups of elements in the periodic table. In general, the Electron gain enthalpy increases from left to right across a period, due to the increasing nuclear charge that attracts the incoming electron. However, there are exceptions to this trend, such as the lower Electron gain enthalpy value of fluorine compared to chlorine, which is attributed to the high electron density of fluorine. The Electron gain enthalpy also tends to become less negative (or more positive) down a group, as the atomic size increases and the attraction between the positively charged nucleus and the incoming electron decreases.
Applications of Electron gain enthalpy
Electron gain enthalpy has numerous practical applications in various areas of chemistry, including materials science, chemical engineering, and environmental science. Some of the major applications of Electron gain enthalpy are:
- Materials Science: Electron gain enthalpy is used in the design and development of new materials with specific electronic and chemical properties. For example, it is used to predict the ability of different elements to form covalent and ionic bonds, which is critical in the development of materials with desired electrical and magnetic properties.
- Chemical Engineering: Electron gain enthalpy is used in chemical engineering to design and optimize chemical processes. By understanding the Electron gain enthalpy of different reactants and products, engineers can predict the energy requirements of a reaction, optimize reaction conditions, and improve overall efficiency.
- Environmental Science: Electron gain enthalpy is used in environmental science to study the behavior of pollutants in the environment. For example, it is used to predict the ability of different pollutants to form chemical bonds with soil and water particles, which can affect their transport and distribution in the environment.
- Biochemistry: Electron gain enthalpy is also relevant to biochemistry, as it helps to explain the reactivity of molecules involved in biological processes, such as enzymes and proteins.
- Predicting Chemical Reactivity: Electron gain enthalpy is used to predict the relative reactivity of different elements and compounds in chemical reactions. For example, a more negative Electron gain enthalpy value indicates a greater tendency to attract an electron and participate in chemical reactions.
Overall, the applications of Electron gain enthalpy are vast and diverse, and its importance in understanding the behavior of atoms and molecules in chemistry cannot be overstated.
