Electronegativity is a measure of the ability of an atom in a molecule to attract electrons towards itself. It is a relative property, which means that it is determined by comparing the electronegativities of different elements. The electronegativity of an element is determined by factors such as the nuclear charge, the number of electrons in the outermost shell, and the distance between the nucleus and the valence electrons.
Electronegativity values are typically assigned using the Pauling scale, which is a scale ranging from 0.7 (for cesium) to 4.0 (for fluorine). Fluorine has the highest electronegativity value because it has a small atomic radius and a high nuclear charge, which allows it to attract electrons strongly. In contrast, elements with low electronegativity values such as cesium have a larger atomic radius and a lower nuclear charge, making them less able to attract electrons.
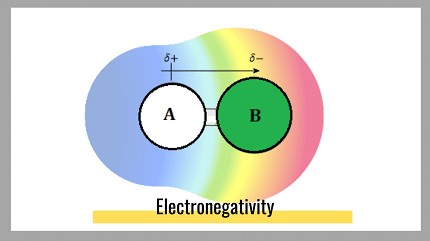
The concept of electronegativity is important in understanding chemical bonding and molecular structure. In covalent bonds, atoms with different electronegativities share electrons unequally, resulting in polar bonds. In ionic bonds, the electronegativity difference between two atoms is so great that one atom completely transfers its electrons to the other atom, resulting in the formation of ions.
What is Required Electronegativity
Required electronegativity, also known as minimum electronegativity difference, is a concept used in chemistry to determine the type of bonding that will occur between two atoms. It refers to the minimum difference in electronegativity between two atoms that is required for them to form a certain type of bond.
For example, if the electronegativity difference between two atoms is less than 0.5, they will form a nonpolar covalent bond because the electrons are shared equally between the atoms. If the electronegativity difference is between 0.5 and 1.7, they will form a polar covalent bond because the electrons are shared unequally between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other atom. If the electronegativity difference is greater than 1.7, they will form an ionic bond, where one atom completely transfers its electrons to the other atom.
The concept of required electronegativity is important in predicting the types of chemical bonds that will form between different elements. It can also help in predicting the properties of molecules and materials based on their bonding types.
When is Required Electronegativity
The concept of Required Electronegativity is used in chemistry to determine the type of chemical bond that will form between two atoms based on the difference in their electronegativities. It is used whenever there is a need to predict the type of bond that will be formed between two elements. This can be in the context of chemical reactions, the formation of molecules or compounds, or the study of the properties of materials.
For example, in the formation of water (H2O), the difference in electronegativity between the hydrogen and oxygen atoms is 1.4, which is greater than the required electronegativity difference of 0.5 for a polar covalent bond. This means that the hydrogen and oxygen atoms will form a polar covalent bond, resulting in a molecule with a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom.
The concept of Required Electronegativity is important in understanding the fundamental principles of chemical bonding and in predicting the properties and behavior of chemical compounds and materials.
Where is Required Electronegativity
The concept of Required Electronegativity is used in the field of chemistry and can be applied in various contexts, such as in the study of chemical reactions, the formation of molecules, and the properties of materials.
It is not a physical entity that can be located in a specific place or location. Rather, it is a concept used to determine the type of bond that will form between two atoms based on their difference in electronegativity. This concept can be applied in various locations, such as in laboratories, classrooms, or research facilities, where chemists and researchers study and explore the properties and behavior of chemical compounds and materials.
How is Required Electronegativity
The Required Electronegativity is determined by calculating the difference between the electronegativity values of two atoms. The electronegativity value of an element is a measure of its ability to attract electrons towards itself when it is in a compound with other elements.
There are various scales used to measure electronegativity, such as the Pauling scale, Mulliken scale, and Allred-Rochow scale. The Pauling scale is the most commonly used and assigns electronegativity values ranging from 0.7 to 4.0 to the elements.
To determine the type of bond that will form between two atoms, the difference between their electronegativity values is calculated. If the difference is less than 0.5, a nonpolar covalent bond will form, if the difference is between 0.5 and 1.7, a polar covalent bond will form, and if the difference is greater than 1.7, an ionic bond will form.
In summary, the Required Electronegativity is calculated by subtracting the electronegativity value of one atom from that of the other atom in a bond or compound, and is used to predict the type of bond that will form between them.
Nomenclature of Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons towards itself in a covalent bond. There are several different scales for measuring electronegativity, but the most commonly used scale is the Pauling scale, which assigns values to each element ranging from 0.7 to 4.0.
The nomenclature of electronegativity typically involves using the name of the scale and the symbol of the element. For example, the Pauling electronegativity value of carbon is 2.55, which can be written as χ(Pauling) = 2.55(C).
In some cases, the difference in electronegativity between two atoms may be used to indicate the type of bond that will form between them, as I described in my previous answer. For example, a bond between carbon and hydrogen in methane (CH4) has a difference in electronegativity of 0.35, which is below the threshold for a polar covalent bond. Therefore, the bond is considered a nonpolar covalent bond.
In summary, the nomenclature of electronegativity involves using the name of the electronegativity scale and the symbol of the element to indicate its electronegativity value, and this value can be used to predict the type of bond that will form between two atoms.
Case Study on Electronegativity
Here’s an example of a case study on electronegativity:
One application of electronegativity is in predicting the properties of molecules and materials based on their bonding types. For instance, water (H2O) has a polar covalent bond between hydrogen and oxygen atoms, where oxygen is more electronegative than hydrogen. This creates a partial negative charge on the oxygen and a partial positive charge on the hydrogen, which gives water its unique properties such as high surface tension, high boiling point, and the ability to dissolve many substances.
On the other hand, methane (CH4) has nonpolar covalent bonds between carbon and hydrogen atoms, where the electronegativity difference between the two atoms is relatively small. This results in an equal sharing of electrons between the atoms, and no partial charges are formed. As a result, methane has different properties compared to water, such as a low boiling point and no ability to dissolve in water.
Another example of how electronegativity affects the properties of molecules is the difference between sodium chloride (NaCl) and water. Sodium chloride is an ionic compound, where sodium has a low electronegativity and chlorine has a high electronegativity. This results in a transfer of electrons from sodium to chlorine, and the formation of an ionic bond. This creates a crystalline structure with high melting and boiling points and the ability to conduct electricity in the molten or aqueous state.
On the other hand, water is a polar covalent compound, where the electronegativity difference between hydrogen and oxygen creates partial charges on the atoms. This creates hydrogen bonds between water molecules, resulting in unique properties such as high boiling point, high specific heat capacity, and the ability to dissolve many substances.
In summary, electronegativity plays an important role in determining the type of bond that forms between atoms, and this in turn affects the properties of molecules and materials. Understanding electronegativity can help us predict and explain the behavior and properties of chemical compounds and materials.
White paper on Electronegativity
Here’s a white paper on Electronegativity:
Introduction:
Electronegativity is a fundamental concept in chemistry that is used to describe the ability of an atom to attract electrons towards itself when it forms a bond with another atom. It is a measure of the electron-attracting power of an atom, and it can be used to predict the type of bond that will form between two atoms, as well as the polarity of the resulting molecule. In this white paper, we will explore the concept of electronegativity in more detail, including its definition, measurement, and applications in chemistry.
Definition:
Electronegativity is a measure of an atom’s ability to attract electrons towards itself when it forms a chemical bond. It is a relative measure, which means that it is based on a comparison between the electronegativity values of two atoms. The higher the electronegativity value of an atom, the more strongly it attracts electrons towards itself when it forms a bond with another atom.
Measurement:
There are several scales used to measure electronegativity, including the Pauling scale, the Mulliken scale, and the Allred-Rochow scale. The most commonly used scale is the Pauling scale, which assigns values ranging from 0.7 to 4.0 to each element.
The Pauling electronegativity values are based on the concept of bond dissociation energy, which is the energy required to break a bond between two atoms in a molecule. The electronegativity value of an atom is calculated as the average of the bond dissociation energies of all the bonds that it forms in a wide range of compounds.
Applications:
Electronegativity has many applications in chemistry. One important application is in predicting the type of bond that will form between two atoms. If the electronegativity difference between two atoms is less than 0.5, a nonpolar covalent bond will form. If the electronegativity difference is between 0.5 and 1.7, a polar covalent bond will form. If the electronegativity difference is greater than 1.7, an ionic bond will form.
Electronegativity is also used to predict the polarity of a molecule. If a molecule has polar covalent bonds, then it will have a dipole moment, which means that one end of the molecule will be partially positive and the other end will be partially negative. This can have important implications for the properties of the molecule, such as its solubility, boiling point, and reactivity.
Conclusion:
In conclusion, electronegativity is a fundamental concept in chemistry that plays an important role in determining the type of bond that will form between two atoms, as well as the polarity of the resulting molecule. It is a relative measure, and it can be measured using various scales, such as the Pauling scale. Electronegativity has many applications in chemistry, including predicting the properties of molecules and materials based on their bonding types. Understanding electronegativity is essential for a deeper understanding of chemical phenomena and for the design and synthesis of new materials with specific properties.