Here are the electronic configurations of the first 36 elements in the periodic table:
- Hydrogen: 1s^1
- Helium: 1s^2
- Lithium: 1s^2 2s^1
- Beryllium: 1s^2 2s^2
- Boron: 1s^2 2s^2 2p^1
- Carbon: 1s^2 2s^2 2p^2
- Nitrogen: 1s^2 2s^2 2p^3
- Oxygen: 1s^2 2s^2 2p^4
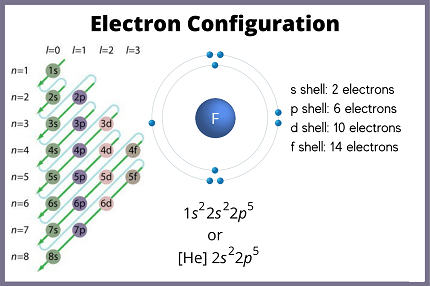
- Fluorine: 1s^2 2s^2 2p^5
- Neon: 1s^2 2s^2 2p^6
- Sodium: 1s^2 2s^2 2p^6 3s^1
- Magnesium: 1s^2 2s^2 2p^6 3s^2
- Aluminum: 1s^2 2s^2 2p^6 3s^2 3p^1
- Silicon: 1s^2 2s^2 2p^6 3s^2 3p^2
- Phosphorus: 1s^2 2s^2 2p^6 3s^2 3p^3
- Sulfur: 1s^2 2s^2 2p^6 3s^2 3p^4
- Chlorine: 1s^2 2s^2 2p^6 3s^2 3p^5
- Argon: 1s^2 2s^2 2p^6 3s^2 3p^6
- Potassium: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1
- Calcium: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2
- Scandium: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^1
- Titanium: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^2
- Vanadium: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^3
- Chromium: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 3d^5
- Manganese: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5
- Iron: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6
- Cobalt: 1s^2 2s^2 2p^
What is Required Electronic configuration of elements
The electronic configuration of an element refers to the arrangement of its electrons in the atomic orbitals of its atoms. This is usually represented using a notation that lists the occupied atomic orbitals and the number of electrons in each. The notation is typically in the form of a series of numbers, letters, and superscripts.
The electronic configuration of an element is required because it determines many of the element’s chemical and physical properties. For example, the valence electrons, or outermost electrons, of an atom are the ones that participate in chemical reactions, and their arrangement in the atomic orbitals of the atom determines the type of chemical bonding that can occur.
The electronic configuration can also provide information about the stability of an atom and its tendency to gain or lose electrons. The periodic table is arranged based on the electronic configuration of the elements, with elements in the same column having similar properties due to their similar valence electron configurations.
Overall, the electronic configuration of an element is an important aspect of its chemical behavior and helps to explain many of the element’s properties.
Who is Required Electronic configuration of elements
The electronic configuration of elements is required by chemists, physicists, and other scientists who study the properties and behavior of elements. It is an important tool in understanding the behavior of atoms in chemical reactions and in the formation of chemical bonds.
Chemists use the electronic configuration of elements to predict the types of chemical bonds that an element can form, as well as the reactivity and stability of its compounds. Physicists use electronic configurations to understand the energy levels of electrons in atoms and to explain the spectral lines of elements.
In addition to scientists, students of chemistry and physics also need to learn and understand the electronic configuration of elements. It is a fundamental concept in these subjects and is often introduced early in high school or college-level chemistry courses.
Overall, the electronic configuration of elements is an important concept for anyone studying or working in the fields of chemistry or physics.
When is Required Electronic configuration of elements
The electronic configuration of elements is required in many different contexts in chemistry and physics. Here are a few examples of when it might be required:
- Predicting chemical properties: The electronic configuration of an element can give clues to its reactivity, stability, and ability to form chemical bonds. Chemists use this information to predict the properties of elements and their compounds.
- Understanding periodic trends: The periodic table is arranged based on the electronic configuration of elements, with elements in the same column having similar valence electron configurations. By understanding the electronic configuration of elements, chemists can explain trends in properties such as atomic radius, ionization energy, and electronegativity across a row or down a column.
- Explaining spectral lines: The electronic configuration of an atom determines the energy levels of its electrons, and these energy levels give rise to the spectral lines observed in atomic spectra. Physicists use electronic configurations to explain the origin of these spectral lines and to determine the electronic transitions that produce them.
- Describing chemical reactions: Chemical reactions involve the transfer or sharing of electrons between atoms. By understanding the electronic configurations of the atoms involved, chemists can predict the types of chemical reactions that will occur and the products that will be formed.
Overall, the electronic configuration of elements is required whenever a deeper understanding of the behavior of atoms and their interactions is needed. It is a fundamental concept in chemistry and physics that has many important applications.
Where is Required Electronic configuration of elements
The electronic configuration of elements can be found in various resources and references, including textbooks, online databases, and periodic tables.
One common place to find the electronic configuration of an element is on a periodic table. Periodic tables often list the electronic configuration of each element along with other important information such as atomic number, symbol, and atomic mass.
Another resource for finding electronic configurations is chemistry textbooks or online databases. These resources provide detailed information about the electronic structure of elements, including the number of electrons in each subshell, the energy level of the valence electrons, and how the electrons are distributed among the atomic orbitals.
It’s important to note that the electronic configuration of elements can vary depending on their ionization state. For example, the electronic configuration of sodium (Na) in its neutral state is 1s² 2s² 2p⁶ 3s¹, but when it loses an electron to become a Na+ ion, its electronic configuration becomes 1s² 2s² 2p⁶. In such cases, it’s important to specify the ionization state of the element to determine its electronic configuration accurately.
In summary, the electronic configuration of elements can be found in a variety of resources, including periodic tables, textbooks, and online databases, and is an essential concept for understanding the behavior of atoms and molecules in chemistry and physics.
How is Required Electronic configuration of elements
The electronic configuration of elements is determined based on the distribution of electrons in the atomic orbitals of their atoms. The electronic configuration follows certain rules and principles based on the behavior of electrons and the properties of the atomic orbitals. Here is a brief overview of how the electronic configuration of elements is determined:
- Aufbau principle: This principle states that electrons occupy the lowest energy orbitals available first, starting with the 1s orbital and progressing through the higher energy orbitals. For example, the electronic configuration of carbon (C) is 1s² 2s² 2p² because the two electrons fill the 1s orbital first, then the 2s orbital, and finally the 2p orbital.
- Pauli exclusion principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, ms). In other words, each orbital can hold a maximum of two electrons with opposite spins. For example, in the carbon atom, the two electrons in the 2p orbital have opposite spins.
- Hund’s rule: This rule states that when electrons occupy degenerate (equal energy) orbitals, they first fill singly with parallel spins before pairing up. For example, in the carbon atom, the two electrons in the 2p orbital are unpaired and have parallel spins.
- Periodic trends: The electronic configuration of elements follows certain periodic trends, with elements in the same column (group) having similar valence electron configurations. For example, all elements in Group 1 (alkali metals) have a valence electron configuration of ns¹, while elements in Group 18 (noble gases) have a valence electron configuration of ns²np⁶.
Overall, the electronic configuration of elements is determined based on these principles and trends. It is a fundamental concept in chemistry and physics that helps explain the behavior of atoms and their interactions in chemical reactions.
Case Study on Electronic configuration of elements
Here is a case study on the electronic configuration of elements:
Case Study: Electronic Configuration of Nitrogen (N)
Nitrogen is a non-metallic element with the symbol N and atomic number 7. Its electronic configuration can be determined by following the rules and principles mentioned earlier.
Step 1: Determine the total number of electrons in the nitrogen atom. Since nitrogen has an atomic number of 7, it has 7 electrons.
Step 2: Fill the electrons into the atomic orbitals in order of increasing energy. Following the Aufbau principle, the first two electrons will occupy the 1s orbital, and the next two will occupy the 2s orbital. The remaining three electrons will occupy the 2p orbitals. According to Hund’s rule, the three 2p orbitals will each have one electron with parallel spins before any of them are paired.
Step 3: Write the electronic configuration using the noble gas shorthand notation. The noble gas shorthand notation represents the electron configuration of the closest preceding noble gas in brackets, followed by the configuration of the valence electrons. The noble gas closest to nitrogen is helium (He), with an electronic configuration of 1s². Therefore, the noble gas shorthand notation for nitrogen is [He] 2s² 2p³.
The electronic configuration of nitrogen shows that it has five valence electrons in the outermost shell, with three of them occupying the 2p orbitals. This arrangement of electrons gives nitrogen unique chemical properties, such as its ability to form covalent bonds with other elements, as it can share electrons with other atoms to achieve a stable configuration. The electronic configuration of nitrogen also explains why it is often found in molecules with other nitrogen atoms, such as N₂, as the sharing of electrons between nitrogen atoms allows both atoms to achieve a stable configuration.
In summary, the electronic configuration of elements is a fundamental concept in chemistry that helps explain the behavior of atoms and their interactions in chemical reactions. The case study of nitrogen demonstrates how electronic configuration can be determined and how it relates to the chemical properties of elements.
White paper on Electronic configuration of elements
Electronic configuration of elements is a fundamental concept in chemistry that helps explain the behavior of atoms and their interactions in chemical reactions. The electronic configuration of an element is the distribution of electrons in the atomic orbitals of its atoms. It is determined based on the principles of quantum mechanics and the behavior of electrons in the atom.
The electronic configuration of an atom follows the Aufbau principle, which states that electrons occupy the lowest energy orbitals first. The orbitals are filled in order of increasing energy, starting with the 1s orbital, then the 2s and 2p orbitals, followed by the 3s, 3p, and 3d orbitals, and so on. Each orbital can hold a maximum of two electrons with opposite spins, according to the Pauli exclusion principle. Hund’s rule states that when electrons occupy degenerate (equal energy) orbitals, they first fill singly with parallel spins before pairing up.
The electronic configuration of an element can be written in several ways. The full electron configuration lists all the electrons in each orbital, while the noble gas shorthand notation represents the electron configuration of the closest preceding noble gas in brackets, followed by the configuration of the valence electrons. The valence electrons are the electrons in the outermost shell of an atom and are responsible for the chemical properties of an element.
The electronic configuration of elements follows certain periodic trends. Elements in the same column (group) of the periodic table have similar valence electron configurations, which give them similar chemical properties. For example, all elements in Group 1 (alkali metals) have a valence electron configuration of ns¹, while elements in Group 18 (noble gases) have a valence electron configuration of ns²np⁶.
The electronic configuration of elements plays a crucial role in explaining the behavior of atoms and their interactions in chemical reactions. It determines the reactivity of elements and their ability to form chemical bonds with other elements. For example, elements with one or two valence electrons tend to lose them to form cations, while elements with five or more valence electrons tend to gain electrons to form anions. The electronic configuration of elements also helps explain the properties of molecules and compounds, such as their shape, polarity, and reactivity.
In conclusion, the electronic configuration of elements is a fundamental concept in chemistry that helps explain the behavior of atoms and their interactions in chemical reactions. It is determined based on the principles of quantum mechanics and the behavior of electrons in the atom. The electronic configuration of elements follows certain periodic trends and plays a crucial role in determining the reactivity and properties of elements, as well as the properties of molecules and compounds.
