Benzene undergoes electrophilic substitution reactions due to its electron-rich nature. The pi electrons of the aromatic ring form a cloud of electron density above and below the plane of the ring, making it susceptible to attack by electrophiles. Some of the common electrophilic substitution reactions of benzene are:
- Nitration: Benzene reacts with nitric acid in the presence of a strong acid catalyst to form nitrobenzene. The electrophile in this reaction is the nitronium ion (NO2+).
- Halogenation: Benzene reacts with halogens (chlorine, bromine, or iodine) in the presence of a Lewis acid catalyst to form the corresponding halobenzene. The electrophile in this reaction is the halogen cation (X+).
- Sulfonation: Benzene reacts with sulfuric acid in the presence of a strong acid catalyst to form benzenesulfonic acid. The electrophile in this reaction is the sulfur trioxide (SO3) molecule, which reacts with water to form the electrophilic species, the sulfonate ion (HSO4-).
- Friedel-Crafts alkylation: Benzene reacts with alkyl halides in the presence of a Lewis acid catalyst (such as aluminum chloride) to form alkyl-substituted benzenes. The electrophile in this reaction is the carbocation intermediate formed by the reaction of the alkyl halide with the Lewis acid catalyst.
- Friedel-Crafts acylation: Benzene reacts with acyl halides in the presence of a Lewis acid catalyst (such as aluminum chloride) to form acyl-substituted benzenes. The electrophile in this reaction is the acylium ion (RCO+), which is formed by the reaction of the acyl halide with the Lewis acid catalyst.
What is Required Benzene Electrophilic substitution reactions
In order for electrophilic substitution reactions to occur with benzene, a few requirements must be met:
- Presence of a strong electron-withdrawing group (EWG) or a catalyst: The presence of a strong electron-withdrawing group (such as a nitro group) or a catalyst (such as aluminum chloride) is necessary to generate the electrophile that will attack the electron-rich benzene ring.
- Electron-rich benzene ring: Benzene must be electron-rich due to the presence of its pi electrons in the aromatic ring. This makes it susceptible to attack by electrophiles.
- Lack of substituents on the benzene ring: The presence of substituents on the benzene ring can influence the reactivity of the ring and may affect the position of substitution. Therefore, the absence of substituents on the benzene ring is preferred for easy and predictable substitution.
- Appropriate reaction conditions: Different electrophilic substitution reactions have different optimal reaction conditions, including temperature, solvent, and catalyst. Appropriate reaction conditions must be selected to achieve high yields and avoid unwanted side reactions.
When is Required Benzene Electrophilic substitution reactions
Benzene electrophilic substitution reactions are useful in the synthesis of a wide variety of organic compounds. They are commonly used in the production of fragrances, dyes, and pharmaceuticals. Some examples include:
- Production of nitrobenzene, which is used as an intermediate in the production of aniline, a key ingredient in the manufacture of dyes and pharmaceuticals.
- Production of chlorobenzene, which is used as a solvent and as an intermediate in the production of other chemicals.
- Production of benzenesulfonic acid, which is used in the manufacture of detergents, dyes, and pharmaceuticals.
- Production of alkyl- and acyl-substituted benzenes, which are used as intermediates in the synthesis of a wide variety of organic compounds.
- Production of phenol, which is used in the manufacture of plastics, pharmaceuticals, and other chemicals.
Overall, benzene electrophilic substitution reactions are important tools in organic synthesis and are used in a wide variety of industrial and academic applications.
Where is Required Benzene Electrophilic substitution reactions
Benzene electrophilic substitution reactions are used in various industries where the production of organic compounds is necessary. Some of the industries where these reactions are commonly used include:
- Chemical industry: The chemical industry uses benzene electrophilic substitution reactions to produce a wide range of organic compounds, including solvents, detergents, pharmaceuticals, fragrances, and dyes.
- Pharmaceutical industry: The pharmaceutical industry uses benzene electrophilic substitution reactions to synthesize complex organic molecules that have therapeutic properties.
- Petrochemical industry: The petrochemical industry uses benzene electrophilic substitution reactions to produce intermediates that are used in the manufacture of plastics, synthetic fibers, and other materials.
- Agrochemical industry: The agrochemical industry uses benzene electrophilic substitution reactions to produce pesticides, herbicides, and other chemicals used in agriculture.
- Research and development: Benzene electrophilic substitution reactions are also used in academic and industrial research laboratories to study and develop new organic compounds.
Overall, benzene electrophilic substitution reactions are used in many industries and applications where the production of organic compounds is necessary.
How is Required Benzene Electrophilic substitution reactions
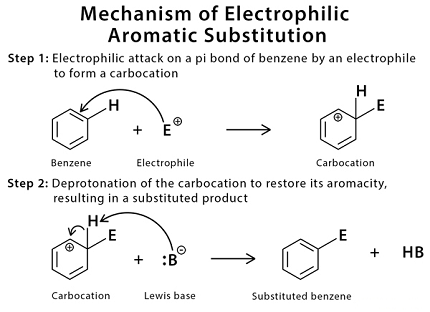
Benzene electrophilic substitution reactions involve the substitution of a hydrogen atom on the benzene ring with an electrophilic species. The general mechanism involves the following steps:
- Generation of the electrophile: A strong electron-withdrawing group (EWG) or a catalyst is used to generate the electrophile that will attack the electron-rich benzene ring. For example, nitric acid and sulfuric acid can be used to generate the nitronium ion and sulfonate ion, respectively. Alternatively, Lewis acid catalysts such as aluminum chloride can be used to generate carbocation or acylium ion electrophiles.
- Formation of the arenium ion: The electrophile attacks the electron-rich benzene ring, forming an intermediate called the arenium ion or sigma complex. This intermediate has a positive charge on the carbon atom that was attacked by the electrophile.
- Rearrangement: The arenium ion can undergo a rearrangement, such as a methyl shift or a hydride shift, to form a more stable intermediate.
- Deprotonation: A proton is removed from the intermediate, resulting in the formation of the substituted benzene product.
Overall, benzene electrophilic substitution reactions are important reactions in organic chemistry and are used in the synthesis of a wide range of organic compounds. The reaction conditions and mechanism may vary depending on the specific electrophile used and the desired substitution product.
Production of Benzene Electrophilic substitution reactions
Benzene electrophilic substitution reactions can be produced in a laboratory setting using a variety of different reagents and conditions, depending on the specific electrophile and desired substitution product. Here are some examples of commonly used electrophiles and reaction conditions:
- Nitration: Benzene can be nitrated by mixing it with a mixture of nitric acid and sulfuric acid. The nitronium ion, generated in situ from the nitric acid, acts as the electrophile that attacks the benzene ring, leading to the formation of nitrobenzene.
- Halogenation: Benzene can be halogenated by mixing it with a halogen (e.g. chlorine or bromine) in the presence of a Lewis acid catalyst (e.g. iron or aluminum chloride). The halogen electrophile attacks the benzene ring, leading to the formation of a halobenzene.
- Friedel-Crafts alkylation: Benzene can be alkylated using an alkyl halide and a Lewis acid catalyst (e.g. aluminum chloride). The alkyl halide electrophile attacks the benzene ring, leading to the formation of an alkylbenzene.
- Friedel-Crafts acylation: Benzene can be acylated using an acyl halide and a Lewis acid catalyst (e.g. aluminum chloride). The acyl halide electrophile attacks the benzene ring, leading to the formation of an acylbenzene.
- Sulfonation: Benzene can be sulfonated by mixing it with sulfuric acid. The sulfonate ion, generated in situ from the sulfuric acid, acts as the electrophile that attacks the benzene ring, leading to the formation of benzenesulfonic acid.
Overall, benzene electrophilic substitution reactions can be produced using a variety of different reagents and conditions, and can be used to synthesize a wide range of organic compounds.
Case Study on Benzene Electrophilic substitution reactions
One example of a case study involving benzene electrophilic substitution reactions is the synthesis of paracetamol (acetaminophen), a common pain reliever and fever reducer. Paracetamol is synthesized via a series of steps that involve benzene electrophilic substitution reactions.
The first step in the synthesis of paracetamol involves the nitration of benzene to form nitrobenzene. This is achieved by mixing benzene with a mixture of nitric acid and sulfuric acid, which generates the nitronium ion as the electrophile. The nitro group on nitrobenzene is then reduced using tin and hydrochloric acid to form aniline.
The second step in the synthesis of paracetamol involves the acylation of aniline using acetic anhydride and an acid catalyst such as sulfuric acid. This reaction forms N-acetylaniline, which is then hydrolyzed using sodium hydroxide to form paracetamol.
Overall, the synthesis of paracetamol involves the use of benzene electrophilic substitution reactions to form nitrobenzene and N-acetylaniline. These reactions highlight the importance of benzene electrophilic substitution reactions in the synthesis of complex organic compounds, particularly those with therapeutic properties.
White paper on Benzene Electrophilic substitution reactions
Benzene electrophilic substitution reactions are an important class of reactions in organic chemistry, as they allow for the substitution of a hydrogen atom on the benzene ring with an electrophilic species, leading to the formation of a substituted benzene product. These reactions are widely used in the synthesis of a variety of organic compounds, including pharmaceuticals, agrochemicals, and polymers.
The mechanism of benzene electrophilic substitution reactions involves the generation of an electrophile that attacks the electron-rich benzene ring, leading to the formation of an arenium ion intermediate. This intermediate can undergo rearrangement and deprotonation steps to form the final substituted product. The choice of electrophile and reaction conditions can be used to control the regioselectivity and stereochemistry of the reaction.
Some common examples of benzene electrophilic substitution reactions include nitration, halogenation, Friedel-Crafts alkylation and acylation, and sulfonation. These reactions can be carried out under a variety of conditions, and the choice of reaction conditions can affect the yield and selectivity of the reaction.
Benzene electrophilic substitution reactions have a wide range of applications in the chemical industry. For example, nitration of benzene is used in the production of nitrobenzene, which is an important intermediate in the synthesis of aniline, phenol, and other organic compounds. Halogenation of benzene is used in the production of chlorobenzene and other halobenzenes, which are used as solvents, intermediates, and starting materials for other reactions. Friedel-Crafts alkylation and acylation reactions are used in the production of alkylbenzenes and acylbenzenes, which are used as solvents, fuels, and intermediates in the synthesis of other organic compounds. Sulfonation of benzene is used in the production of benzenesulfonic acid and other sulfonated compounds, which are used as detergents, surfactants, and catalysts.
However, benzene electrophilic substitution reactions can also have negative environmental and health impacts, as benzene is a known carcinogen and can cause serious health effects even at low levels of exposure. Therefore, it is important to use appropriate safety measures and environmentally-friendly reaction conditions when carrying out these reactions.
In conclusion, benzene electrophilic substitution reactions are an important class of reactions in organic chemistry that are widely used in the chemical industry. These reactions allow for the synthesis of a variety of important organic compounds, but also require careful consideration of safety and environmental impacts.
