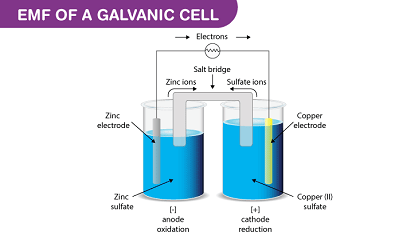
A galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy. The cell consists of two electrodes, each with a different reduction potential, that are connected by an electrolyte. The potential difference between the two electrodes is known as the cell potential or electromotive force (EMF) of the cell.
The EMF of a galvanic cell is a measure of the cell’s ability to generate an electric current. It is the difference in the reduction potentials of the two electrodes, measured in volts (V). The reduction potential is a measure of the tendency of an electrode to gain electrons and reduce, and it is affected by factors such as the nature of the electrode material, the concentration of the electrolyte, and the temperature.
The EMF of a galvanic cell can be calculated using the Nernst equation, which takes into account the standard reduction potentials of the two electrodes, the concentrations of the ions in the electrolyte, and the temperature. The Nernst equation is:
Ecell = E°cell – (RT/nF)lnQ
where: Ecell is the cell potential or EMF E°cell is the standard cell potential or EMF, which is the cell potential when the concentrations of all species are at their standard state R is the gas constant (8.314 J/mol·K) T is the temperature in Kelvin n is the number of electrons transferred in the balanced equation for the cell reaction F is Faraday’s constant (96,485 C/mol) Q is the reaction quotient, which is the ratio of the concentrations of the products to the concentrations of the reactants, each raised to the power of its stoichiometric coefficient.
In general, the higher the EMF of a galvanic cell, the greater the potential difference between the electrodes and the greater the electrical energy that can be generated.
What is Required Emf of galvanic cells
The required EMF (electromotive force) of a galvanic cell depends on the specific application and the desired output of the cell.
For example, in a battery-powered device, such as a flashlight, the required EMF of the galvanic cell would be determined by the voltage needed to power the device. In general, the cells used in batteries are designed to provide a specific voltage, such as 1.5 volts for a standard AA battery, and are typically composed of multiple cells connected in series to provide the required voltage.
In other applications, such as electroplating or corrosion protection, the required EMF of the galvanic cell is determined by the specific reaction that needs to take place. For example, in electroplating, the required EMF is determined by the voltage needed to drive the desired reduction reaction on the surface of the object being plated.
In some cases, the required EMF can be achieved by a single galvanic cell, while in other cases, multiple cells may need to be connected in series or parallel to provide the necessary voltage and current. In general, the required EMF of a galvanic cell is determined by the specific application and the electrical requirements of the system in which it will be used.
Who is Required Emf of galvanic cells
“Required EMF of galvanic cells” is a technical term used in the field of electrochemistry to refer to the minimum amount of electromotive force (EMF) needed for a galvanic cell to perform a specific function or application.
This term is relevant in a wide range of practical applications of galvanic cells, such as batteries, electroplating, corrosion protection, and many others. In each case, the required EMF depends on the specific requirements of the application, such as the voltage needed to power a device, the voltage required to drive a specific electrochemical reaction, or the voltage needed to prevent corrosion in a particular environment.
In general, the required EMF of a galvanic cell is determined by the specific application and the electrical requirements of the system in which it will be used. It is an important consideration in the design and selection of galvanic cells for a wide range of practical applications.
When is Required Emf of galvanic cells
The concept of “required EMF of galvanic cells” is applicable in a wide range of situations where galvanic cells are used to generate electrical energy or perform electrochemical reactions. Some examples of when the required EMF of galvanic cells may be relevant include:
- Battery-powered devices: The required EMF of a battery is determined by the voltage required to power the device it is intended to operate. For example, a standard AA battery has a nominal voltage of 1.5 volts, which is sufficient to power many common devices.
- Electroplating: In electroplating, a galvanic cell is used to deposit a layer of metal onto a substrate. The required EMF of the cell is determined by the voltage needed to drive the desired reduction reaction on the surface of the object being plated.
- Corrosion protection: Galvanic cells can be used to prevent corrosion of metal structures in harsh environments. The required EMF of the cell is determined by the voltage needed to create a protective potential difference between the metal structure and the electrolyte solution.
- Fuel cells: Fuel cells use a galvanic reaction to generate electrical energy from a fuel source, such as hydrogen or methanol. The required EMF of the cell is determined by the voltage needed to drive the electrochemical reaction and generate a usable amount of electrical energy.
In general, the required EMF of galvanic cells is determined by the specific application and the electrical requirements of the system in which they are used. It is an important consideration in the design and selection of galvanic cells for many practical applications.
Where is Required Emf of galvanic cells
The concept of “required EMF of galvanic cells” is relevant in a wide range of fields and applications where galvanic cells are used to generate electrical energy or perform electrochemical reactions.
Some examples of where the required EMF of galvanic cells may be important include:
- Battery-powered devices: In many portable electronic devices, such as cell phones, laptops, and flashlights, galvanic cells are used as a power source. The required EMF of the cell is determined by the voltage needed to power the device.
- Industrial processes: Galvanic cells are used in a variety of industrial processes, such as electroplating, metal refining, and corrosion protection. The required EMF of the cell is determined by the specific requirements of the process, such as the voltage needed to drive a specific electrochemical reaction or prevent corrosion.
- Renewable energy systems: Galvanic cells are used in many renewable energy systems, such as solar panels and fuel cells. The required EMF of the cell is determined by the voltage needed to generate usable electrical energy from the available energy source.
- Medical devices: Galvanic cells are used in a variety of medical devices, such as pacemakers and hearing aids. The required EMF of the cell is determined by the voltage needed to power the device and ensure reliable operation.
In general, the required EMF of galvanic cells is determined by the specific application and the electrical requirements of the system in which they are used. It is an important consideration in the design and selection of galvanic cells for many practical applications.
How is Required Emf of galvanic cells
The required EMF (electromotive force) of galvanic cells is determined by the specific application and the electrical requirements of the system in which they are used. The EMF of a galvanic cell is related to the standard electrode potentials of the two half-cells that make up the cell.
The standard electrode potential is a measure of the tendency of a half-cell to gain or lose electrons, and is expressed in volts. The EMF of a galvanic cell is the difference between the standard electrode potentials of the two half-cells.
For example, in a simple galvanic cell consisting of a zinc electrode and a copper electrode, the standard electrode potential of the zinc electrode is -0.76 volts, while the standard electrode potential of the copper electrode is +0.34 volts. The EMF of the cell is therefore calculated as:
EMF = E(copper) – E(zinc) = +0.34 V – (-0.76 V) = +1.10 V
The required EMF of a galvanic cell for a specific application is determined by the voltage needed to power the device, drive a specific electrochemical reaction, or achieve a specific potential difference between the electrodes and the electrolyte.
In many cases, the required EMF can be achieved by a single galvanic cell, while in other cases, multiple cells may need to be connected in series or parallel to provide the necessary voltage and current.
In summary, the required EMF of galvanic cells is determined by the specific application and the electrical requirements of the system in which they will be used, and is related to the standard electrode potentials of the two half-cells that make up the cell.
Case Study on Emf of galvanic cells
Case Study: The use of galvanic cells in automotive batteries
Galvanic cells, also known as electrochemical cells, are used in a wide range of applications, including batteries. One of the most common applications of galvanic cells is in automotive batteries, which are used to provide electrical energy to start the engine and power various electrical systems in the vehicle.
In an automotive battery, a galvanic cell consisting of two electrodes (usually lead and lead dioxide) and an electrolyte (usually sulfuric acid) is used to generate a voltage and provide electrical energy. The voltage of the battery, also known as the EMF, is determined by the standard electrode potential of the half-cells that make up the battery.
The EMF of an automotive battery is typically around 12 volts, which is sufficient to power the electrical systems in most vehicles. However, the actual voltage of the battery can vary depending on a range of factors, including the temperature, the state of charge of the battery, and the load on the battery.
The performance of an automotive battery is also affected by a range of other factors, such as the number of cells in the battery, the size and construction of the electrodes, and the composition of the electrolyte. In addition, the design and construction of the battery must also take into account factors such as safety, durability, and cost.
In recent years, there has been increasing interest in the development of new types of automotive batteries that use alternative electrode materials and electrolytes to improve performance and reduce costs. Some examples of these new battery technologies include lithium-ion batteries, nickel-metal hydride batteries, and solid-state batteries.
Despite the development of new battery technologies, galvanic cells remain an essential component of automotive batteries, providing a reliable and cost-effective source of electrical energy for vehicles. As the automotive industry continues to evolve and new technologies emerge, the use of galvanic cells in automotive batteries is likely to remain an important area of research and development.
White paper on Emf of galvanic cells
Introduction
Galvanic cells, also known as electrochemical cells, are devices that convert chemical energy into electrical energy. They are widely used in a range of applications, including batteries, fuel cells, corrosion protection, and electroplating. In this white paper, we will focus on the EMF (electromotive force) of galvanic cells, including its definition, measurement, and applications.
Definition
The EMF of a galvanic cell is the difference in potential between the two electrodes in the cell. It is the driving force that causes electrons to flow from the anode (negative electrode) to the cathode (positive electrode) through an external circuit. The EMF is a measure of the tendency of the two half-cells to undergo an electrochemical reaction and is related to the standard electrode potentials of the two half-cells.
Measurement
The EMF of a galvanic cell can be measured using a voltmeter. The voltmeter is connected to the anode and cathode of the cell, and the voltage difference between the two electrodes is measured. The measured voltage is equal to the EMF of the cell if there is no current flowing through the cell.
Applications
Galvanic cells are used in a wide range of applications, including batteries, fuel cells, and electroplating. In batteries, galvanic cells are used to provide a source of electrical energy. The EMF of the battery determines the voltage of the battery, which in turn determines the amount of electrical energy that can be stored in the battery.
In fuel cells, galvanic cells are used to convert the chemical energy in fuels such as hydrogen and methanol into electrical energy. The EMF of the fuel cell determines the efficiency of the fuel cell, and higher EMF values lead to higher efficiency.
In electroplating, galvanic cells are used to deposit metal onto a substrate. The EMF of the cell determines the rate of deposition, and higher EMF values lead to faster deposition rates.
Conclusion
In conclusion, the EMF of galvanic cells is an important parameter that determines the performance of galvanic cells in a range of applications. It is a measure of the driving force that causes electrons to flow through an external circuit from the anode to the cathode. The EMF of galvanic cells can be measured using a voltmeter, and it is related to the standard electrode potentials of the two half-cells in the cell.
