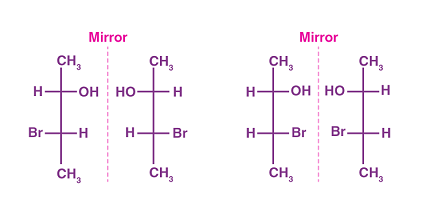
Enantiomers are a type of stereoisomerism that occurs in organic chemistry when two molecules are non-superimposable mirror images of each other. They have the same chemical and physical properties, except for their effect on plane-polarized light and interactions with other chiral molecules.
Enantiomers have a chiral center, which is an atom that is attached to four different groups. The arrangement of these groups around the chiral center determines whether the molecule is a left-handed or right-handed enantiomer. They are named using the R/S nomenclature, which is based on the priority of the groups around the chiral center.
Enantiomers are important in many fields, including biochemistry and pharmacology, where they can have different biological activities and effects on the body. For example, the drug thalidomide was originally developed as a sedative, but one enantiomer caused birth defects, while the other had therapeutic effects. As a result, enantiomerically pure drugs are often preferred to ensure their safety and efficacy.
What is Required Basic Principles of Organic Chemistry Enantiomers
Enantiomers are a fundamental concept in organic chemistry and are stereoisomers that are non-superimposable mirror images of each other. The following are the required basic principles of organic chemistry for enantiomers:
- Chirality: Enantiomers have chirality, which means that they lack internal symmetry and cannot be superimposed on their mirror image.
- Mirror Images: Enantiomers exist as pairs of molecules that are mirror images of each other, similar to how your left and right hands are mirror images of each other.
- Stereochemistry: Enantiomers have the same molecular formula and structure but differ in the arrangement of atoms or groups in space.
- Optical Activity: Enantiomers have different optical activities, meaning they rotate plane-polarized light in opposite directions.
- Naming Convention: Enantiomers are named using the R/S nomenclature, which is based on the orientation of priority groups around a chiral center.
- Physical and Chemical Properties: Enantiomers have identical physical and chemical properties, except for their effect on plane-polarized light and interactions with other chiral molecules.
Understanding the basic principles of enantiomers is crucial in organic chemistry because it has significant implications for biological systems, drug design, and chemical synthesis.
When is Required Basic Principles of Organic Chemistry Enantiomers
The required basic principles of organic chemistry for enantiomers are relevant in various areas of chemistry and related fields, including:
- Drug Development: Enantiomers can have different biological activities and effects on the body, making it essential to determine their stereochemistry during drug development.
- Chemical Synthesis: Enantiomers can be produced during chemical synthesis, and it is important to control their formation to ensure that the final product is enantiomerically pure.
- Biochemistry: Enantiomers play a crucial role in biological systems, such as amino acids, which occur naturally in their L-form and have different properties from their D-form.
- Analytical Chemistry: Enantiomers can be separated and analyzed using various techniques, including chiral chromatography and polarimetry.
Overall, the required basic principles of organic chemistry for enantiomers are relevant in many areas of chemistry and related fields, where a thorough understanding of their stereochemistry is essential for accurate analysis, synthesis, and characterization.
Where is Required Basic Principles of Organic Chemistry Enantiomers
The required basic principles of organic chemistry for enantiomers are applicable in various settings, including academic research, industrial laboratories, pharmaceutical companies, and regulatory agencies.
In academic research, enantiomers are essential for studying the behavior of biological molecules and synthesizing novel chemical compounds. Researchers use the principles of enantiomers to design experiments and interpret their results accurately.
In industrial laboratories, the principles of enantiomers are relevant in the production and testing of chemicals, including drugs and agrochemicals. Industrial chemists work to ensure that their final products are enantiomerically pure and have the desired stereochemistry.
In pharmaceutical companies, enantiomers are crucial in drug development, where researchers must understand their biological activity and effects on the body. Pharmaceutical companies use the principles of enantiomers to develop enantiomerically pure drugs that are safe and effective for patients.
In regulatory agencies, the principles of enantiomers are essential for evaluating the safety and efficacy of drugs and other chemicals. Regulators use the principles of enantiomers to assess the quality, purity, and potency of drugs and to ensure that they comply with regulatory standards.
Overall, the principles of enantiomers are applicable in various settings, where they play an essential role in advancing scientific understanding, improving chemical synthesis, and ensuring the safety and efficacy of drugs and other chemicals.
How is Required Basic Principles of Organic Chemistry Enantiomers
The required basic principles of organic chemistry for enantiomers are based on the properties of stereoisomers, which are molecules that have the same molecular formula and connectivity but differ in their spatial arrangement. Enantiomers are a specific type of stereoisomerism that arises when two molecules are non-superimposable mirror images of each other.
The basic principles for enantiomers include:
- Chirality: Enantiomers lack internal symmetry and cannot be superimposed on their mirror image.
- Stereochemistry: Enantiomers have the same molecular formula and structure but differ in the arrangement of atoms or groups in space.
- Mirror images: Enantiomers exist as pairs of molecules that are mirror images of each other.
- Optical activity: Enantiomers have different optical activities, meaning they rotate plane-polarized light in opposite directions.
- Naming convention: Enantiomers are named using the R/S nomenclature, which is based on the orientation of priority groups around a chiral center.
These principles are essential in determining the stereochemistry of enantiomers, which is important for understanding their biological activity, synthesizing enantiomerically pure compounds, and analyzing their properties using various techniques such as chiral chromatography and polarimetry.
The basic principles of enantiomers are typically taught in introductory organic chemistry courses, and their application is further explored in advanced courses and in research settings. Chemists use these principles to design experiments, synthesize novel compounds, and evaluate the safety and efficacy of drugs and other chemicals.
Production of Basic Principles of Organic Chemistry Enantiomers
Enantiomers can be produced in various ways, including:
- Asymmetric synthesis: This method involves the use of chiral reagents or catalysts to selectively generate one enantiomer over the other. Asymmetric synthesis can be achieved using a variety of techniques, such as chiral auxiliaries, chiral pool synthesis, and asymmetric catalysis.
- Enantioselective chromatography: This technique separates enantiomers based on their interaction with a chiral stationary phase. Enantioselective chromatography is commonly used in the pharmaceutical industry to separate and purify enantiomers.
- Biological processes: Enantiomers can be produced using biological processes, such as enzymatic resolution or fermentation. Enzymatic resolution involves the use of enzymes to selectively break down one enantiomer, leaving the other enantiomer behind. Fermentation involves the use of microorganisms to produce enantiomers.
- Racemization and resolution: Racemization converts a mixture of enantiomers into a racemic mixture, which can then be resolved into individual enantiomers using separation techniques such as crystallization or chromatography.
- Chemical transformation: Enantiomers can be produced through chemical transformation by converting one enantiomer into the other. Chemical transformation can be achieved using a variety of techniques, such as oxidation, reduction, and inversion.
The production of enantiomers is an essential aspect of organic chemistry and plays a crucial role in the development of new drugs, agrochemicals, and materials. The production of enantiomerically pure compounds is often required for these applications, and the choice of production method depends on various factors, including the desired enantiomer, the starting material, and the available resources.
Case Study on Basic Principles of Organic Chemistry Enantiomers
One interesting case study on the basic principles of organic chemistry and enantiomers is the development of the drug thalidomide.
Thalidomide was developed in the late 1950s as a sedative and anti-nausea medication for pregnant women. However, it was soon discovered that the drug caused severe birth defects, such as limb deformities, in babies born to mothers who took the drug during pregnancy.
Investigations into the cause of the birth defects revealed that thalidomide was a chiral molecule and existed as two enantiomers. The drug was initially produced as a racemic mixture, meaning it contained equal amounts of both enantiomers. It was later discovered that one enantiomer was responsible for the therapeutic effects of the drug, while the other enantiomer caused the birth defects.
The discovery of thalidomide’s enantiomers highlighted the importance of stereochemistry in drug development and led to significant changes in drug regulation. Today, drug manufacturers are required to test the stereochemistry of their drugs and provide enantiomerically pure compounds whenever possible.
Further research on thalidomide led to the development of a more potent and safer derivative of the drug, known as lenalidomide. Lenalidomide is a chiral molecule and exists as a single enantiomer. The selective synthesis of this enantiomer was critical in the development of lenalidomide as a successful cancer treatment.
The case of thalidomide underscores the importance of understanding the basic principles of organic chemistry and enantiomers in drug development and underscores the critical role of stereochemistry in drug efficacy and safety.
White paper on Basic Principles of Organic Chemistry Enantiomers
Introduction
Enantiomers are a specific type of stereoisomerism that plays a critical role in various fields of chemistry, including drug development, agrochemicals, and materials science. Enantiomers have the same molecular formula and structure but differ in their spatial arrangement, making them non-superimposable mirror images of each other. The study of enantiomers and their properties is essential in understanding the fundamental principles of organic chemistry and the applications of stereochemistry in different areas of science.
Chirality and Stereochemistry
The basic principles of enantiomers are rooted in the concepts of chirality and stereochemistry. Chirality refers to the lack of internal symmetry in a molecule, and chiral molecules are those that cannot be superimposed on their mirror image. Stereochemistry, on the other hand, is concerned with the spatial arrangement of atoms or groups in a molecule and how this arrangement affects the molecule’s properties.
Mirror Images and Optical Activity
Enantiomers exist as pairs of molecules that are mirror images of each other. The two enantiomers have the same physical and chemical properties, except for their interaction with plane-polarized light. Enantiomers have different optical activities, meaning they rotate plane-polarized light in opposite directions. The specific direction of rotation depends on the absolute configuration of the molecule.
Naming Convention
The R/S nomenclature is commonly used to name enantiomers. The naming convention is based on the orientation of priority groups around a chiral center, with R representing a clockwise arrangement of groups and S representing a counterclockwise arrangement of groups.
Production of Enantiomers
Enantiomers can be produced using various methods, including asymmetric synthesis, enantioselective chromatography, biological processes, racemization and resolution, and chemical transformation. The choice of production method depends on various factors, including the desired enantiomer, the starting material, and the available resources.
Application of Enantiomers
Enantiomers play a crucial role in various fields of chemistry. In drug development, the stereochemistry of a drug can affect its efficacy, toxicity, and side effects. The development of enantiomerically pure drugs is essential in ensuring the safety and efficacy of the drug. In materials science, the properties of enantiomers can affect their mechanical, electrical, and optical properties, making them important for applications such as drug delivery, catalysis, and sensors.
Conclusion
The basic principles of organic chemistry and enantiomers are essential in understanding the properties and applications of stereochemistry in different areas of science. The study of enantiomers has played a critical role in the development of new drugs, agrochemicals, and materials, highlighting the importance of stereochemistry in modern chemistry. The production of enantiomerically pure compounds and the understanding of their properties continue to be an active area of research and development in chemistry.
