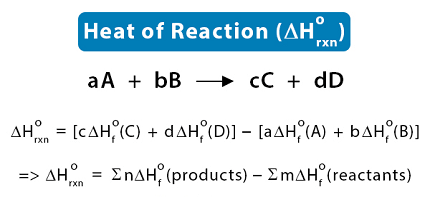
The enthalpy of reaction (ΔHrxn) is the amount of heat released or absorbed during a chemical reaction at a constant pressure. It represents the change in the enthalpy of the system as reactants are transformed into products.
If the enthalpy of reaction is negative (ΔHrxn < 0), it means that the reaction is exothermic, and heat is released into the surroundings. If the enthalpy of reaction is positive (ΔHrxn > 0), it means that the reaction is endothermic, and heat is absorbed from the surroundings.
The enthalpy of reaction can be calculated using Hess’s law or measured experimentally using calorimetry. It is an important thermodynamic property that can help us understand and predict the behavior of chemical reactions.
What is Required Enthalpy of reaction
I believe you may be referring to the concept of the “required enthalpy of reaction” in the context of chemical engineering and process design.
In chemical engineering, the required enthalpy of reaction is the amount of heat energy that needs to be added or removed from a reaction system in order to achieve a desired degree of conversion or yield of the desired product(s). It is a critical parameter in the design and optimization of chemical reaction processes.
The required enthalpy of reaction can be calculated based on the enthalpy change of the reaction (ΔHrxn) and the extent of the reaction that is desired. This calculation takes into account factors such as the temperature and pressure conditions of the reaction, the thermodynamic properties of the reactants and products, and the heat transfer characteristics of the reactor and any heat exchange equipment.
By determining the required enthalpy of reaction, process engineers can select the appropriate reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield.
When is Required Enthalpy of reaction
The concept of “required enthalpy of reaction” is relevant whenever there is a need to design or optimize a chemical reaction process. This could be in industries such as petrochemicals, pharmaceuticals, or food and beverage, where chemical reactions are used to produce desired products on a large scale.
In order to achieve a desired degree of conversion or yield of the desired product(s), it is necessary to carefully control the temperature and heat transfer in the reaction system. This requires an understanding of the enthalpy change of the reaction, as well as the thermodynamic properties of the reactants and products.
The required enthalpy of reaction is calculated based on these factors, and is used to select the appropriate reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield. Therefore, the concept of required enthalpy of reaction is relevant whenever there is a need to design or optimize a chemical reaction process.
Where is Required Enthalpy of reaction
The concept of “required enthalpy of reaction” is used in chemical engineering and process design, where it is applied to the design and optimization of chemical reaction processes. These processes can take place in a variety of locations, including industrial plants, research laboratories, and pilot plants.
The required enthalpy of reaction is an important parameter that must be considered in the design of the reaction system, including the selection of the reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield.
The reaction system itself may be located in a variety of places, depending on the specific application. For example, in the petrochemical industry, the reaction system may be located within a refinery or chemical plant. In the pharmaceutical industry, the reaction system may be located in a laboratory or pilot plant. Regardless of the specific location, the concept of required enthalpy of reaction is an important consideration in the design and optimization of the chemical reaction process.
How is Required Enthalpy of reaction
The “required enthalpy of reaction” is calculated based on the thermodynamic properties of the reactants and products, the enthalpy change of the reaction, and the desired degree of conversion or yield of the desired product(s). The calculation takes into account factors such as the temperature and pressure conditions of the reaction, the heat transfer characteristics of the reactor, and any heat exchange equipment.
The calculation of the required enthalpy of reaction can be complex and may involve iterative calculations or computer simulations. However, in general, the calculation involves the following steps:
- Determine the enthalpy change of the reaction (ΔHrxn) using thermodynamic data or experimental measurements.
- Determine the extent of the reaction that is desired, usually expressed as the degree of conversion or yield of the desired product(s).
- Calculate the heat of reaction (Qrxn) using the enthalpy change and the amount of reactants consumed or products formed.
- Determine the heat transfer coefficient (U) for the reactor and any heat exchange equipment.
- Calculate the required heat transfer area (A) using the equation Q = U * A * ΔT, where ΔT is the temperature difference between the reaction system and the cooling or heating medium.
- Calculate the required enthalpy of reaction as the heat energy that needs to be added or removed from the reaction system to achieve the desired degree of conversion or yield.
The required enthalpy of reaction is an important parameter that must be carefully controlled in the design and operation of chemical reaction processes, as it can have a significant impact on the efficiency, selectivity, and safety of the process.
Nomenclature of Enthalpy of reaction
The nomenclature for enthalpy of reaction, also known as heat of reaction, is denoted by the symbol ΔH_rxn. The ΔH represents the change in enthalpy, while “rxn” denotes the chemical reaction being considered. The ΔH_rxn is expressed in units of energy per mole of reaction, such as joules per mole (J/mol) or kilojoules per mole (kJ/mol).
The enthalpy of reaction can be further categorized depending on the temperature and pressure conditions of the reaction. For instance, the standard enthalpy of reaction, denoted by ΔH°_rxn, represents the enthalpy change of a reaction under standard state conditions of 298 K (25°C) and 1 atm pressure.
In addition, the enthalpy of reaction can be classified as exothermic or endothermic, depending on whether the reaction releases or absorbs heat, respectively. An exothermic reaction has a negative ΔH_rxn, indicating that energy is released during the reaction, while an endothermic reaction has a positive ΔH_rxn, indicating that energy is absorbed during the reaction.
Case Study on Enthalpy of reaction
One example of a case study on the “enthalpy of reaction” could involve the design and optimization of a chemical reaction process for the production of a specific product.
For instance, let’s consider the reaction of ethylene oxide (C2H4O) with water to produce ethylene glycol (C2H6O2), which is a common industrial process in the production of antifreeze, polyester fibers, and plastics. The enthalpy change for this reaction is -32.6 kJ/mol.
Assuming a desired conversion of 80%, we can calculate the required enthalpy of reaction for this process as follows:
- Determine the heat of reaction (Qrxn): Qrxn = (-32.6 kJ/mol) * (0.8 mol) = -26.08 kJ
- Determine the heat transfer coefficient (U): Assuming a U value of 500 W/m2.K for the reactor and heat exchanger system.
- Calculate the required heat transfer area (A): A = Q / (U * ΔT) Assuming a temperature difference of 15°C between the reactor and cooling water, we have: A = (-26.08 kJ) / (500 W/m2.K * 15°C) = 0.00349 m2
- Based on this calculation, we can determine that a reactor and heat exchanger system with a total heat transfer area of 0.00349 m2 will be required to achieve the desired degree of conversion while maintaining the reaction temperature.
This example illustrates how the required enthalpy of reaction is an important factor in the design and optimization of chemical reaction processes. It can be used to determine the appropriate reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield.
White paper on Enthalpy of reaction
Here is a white paper on “Enthalpy of Reaction” which provides an overview of the concept, its importance in chemical engineering, and its applications in the design and optimization of chemical reaction processes.
Introduction
Chemical reactions involve the transformation of one or more chemical species into another. During a chemical reaction, energy is either absorbed or released, and this is reflected in the enthalpy change of the reaction. Enthalpy is a measure of the heat energy contained in a substance, and the enthalpy change of a reaction is the difference in the enthalpy of the reactants and products. The enthalpy change of a reaction is an important parameter in chemical engineering, as it can have a significant impact on the efficiency, selectivity, and safety of the reaction process.
Enthalpy of Reaction in Chemical Engineering
In chemical engineering, the enthalpy of reaction is used to design and optimize chemical reaction processes. It is a key parameter that must be considered in the selection of the reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield.
The required enthalpy of reaction is calculated based on the thermodynamic properties of the reactants and products, the enthalpy change of the reaction, and the desired degree of conversion or yield of the desired product(s). The calculation takes into account factors such as the temperature and pressure conditions of the reaction, the heat transfer characteristics of the reactor, and any heat exchange equipment.
Applications of Enthalpy of Reaction in Chemical Engineering
The concept of enthalpy of reaction is applied in a wide range of chemical engineering applications, including:
- Process Design: The required enthalpy of reaction is used to design and optimize chemical reaction processes. This involves the selection of the appropriate reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield.
- Safety Analysis: The enthalpy change of a reaction is an important parameter in safety analysis, as it can indicate the potential for exothermic reactions and the need for additional cooling or safety measures.
- Energy Conservation: The enthalpy change of a reaction can be used to optimize energy use in chemical reaction processes. For example, exothermic reactions can be used to generate steam, which can be used to power other processes in the plant.
- Environmental Impact: The enthalpy change of a reaction can also be used to assess the environmental impact of chemical reaction processes, particularly in terms of greenhouse gas emissions and energy consumption.
Conclusion
Enthalpy of reaction is a critical parameter in chemical engineering, and it is used to design and optimize chemical reaction processes. By considering the enthalpy change of a reaction, engineers can select the appropriate reactor type and design, as well as the heat transfer equipment necessary to maintain the desired temperature and achieve the desired degree of conversion or yield. As such, enthalpy of reaction is an essential concept in chemical engineering and plays a vital role in the efficient and sustainable production of chemicals and materials.
