Equilibrium constant (Kp and Kc) and Reaction quotient are terms commonly used in the field of chemical equilibrium.
Equilibrium constant (Kp or Kc) is a numerical value that relates the concentrations (or partial pressures) of the reactants and products of a reversible chemical reaction at equilibrium. The equilibrium constant expression is written as:
Kp = (P products)^(coefficients of products) / (P reactants)^(coefficients of reactants) Kc = [products]^ (coefficients of products) / [reactants]^ (coefficients of reactants)
where P represents the partial pressure of a gas and [ ] represents the concentration of a species in mol/L.
The value of Kp or Kc is a constant at a given temperature and does not depend on the initial concentrations of the reactants and products. The magnitude of Kp or Kc indicates the extent to which a reaction proceeds to the right or left at equilibrium.
The Reaction quotient (Q) is a similar expression to the equilibrium constant (Kp or Kc), except that it is calculated at any time during a chemical reaction and not only at equilibrium. The reaction quotient allows us to determine whether a reaction is proceeding towards the formation of products or reactants at a particular moment.
Q = (P products)^(coefficients of products) / (P reactants)^(coefficients of reactants) or [products]^ (coefficients of products) / [reactants]^ (coefficients of reactants)
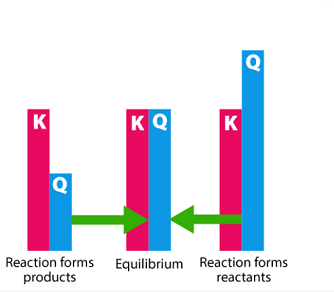
If the value of Q is greater than K, the reaction proceeds in the reverse direction to form reactants, and if Q is less than K, the reaction proceeds in the forward direction to form products. If Q is equal to K, the reaction is at equilibrium.
What is Required Equilibrium constant (Kp and Kc) and Reaction quotient
The Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are numerical values used to describe the extent to which a reversible chemical reaction proceeds to the right or left at a given temperature.
The Equilibrium Constant (Kp or Kc) is a constant that describes the ratio of the concentrations or partial pressures of products and reactants at equilibrium, and is expressed as:
Kp = (P products)^(coefficients of products) / (P reactants)^(coefficients of reactants) Kc = [products]^ (coefficients of products) / [reactants]^ (coefficients of reactants)
where P represents the partial pressure of a gas and [ ] represents the concentration of a species in mol/L.
The Reaction Quotient (Q) is similar to the equilibrium constant, but it is calculated using the initial concentrations or partial pressures of reactants and products at any time during the reaction, and is expressed as:
Q = (P products)^(coefficients of products) / (P reactants)^(coefficients of reactants) or [products]^ (coefficients of products) / [reactants]^ (coefficients of reactants)
If the value of Q is less than K, the reaction proceeds to the right to form more products until equilibrium is reached. If the value of Q is greater than K, the reaction proceeds to the left to form more reactants until equilibrium is reached. If the value of Q is equal to K, the system is at equilibrium.
The Required Equilibrium Constant and Reaction Quotient are important concepts in chemical equilibrium, as they allow us to predict the direction and extent of a chemical reaction under different conditions.
When is Required Equilibrium constant (Kp and Kc) and Reaction quotient
The Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are used in the study of chemical equilibrium, which occurs when a chemical reaction is reversible, meaning that reactants can be converted into products, and products can be converted back into reactants. Equilibrium occurs when the rate of the forward reaction is equal to the rate of the reverse reaction, and the concentrations or partial pressures of the reactants and products no longer change over time.
The Equilibrium Constant (Kp and Kc) and Reaction Quotient are used to describe the position of the equilibrium and the extent to which a reaction proceeds in either the forward or reverse direction. These values are used in many applications, such as the design and optimization of industrial processes, the analysis of environmental systems, and the study of biological systems. Therefore, the Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are used whenever a chemical reaction is reversible and the position of the equilibrium needs to be determined.
Where is Required Equilibrium constant (Kp and Kc) and Reaction quotient
The Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are concepts used in the field of chemistry to describe the behavior of reversible chemical reactions. They are not physical entities that exist in a specific location, but rather they are mathematical values that describe the concentrations or partial pressures of reactants and products at a given temperature and pressure.
The Equilibrium Constant (Kp and Kc) and Reaction Quotient are typically calculated and used in laboratory experiments, industrial processes, and theoretical modeling. They are also used in many applications, such as the design and optimization of industrial processes, the analysis of environmental systems, and the study of biological systems. Therefore, the Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are used in various locations, including laboratories, industrial plants, research institutions, and academic institutions.
Nomenclature of Equilibrium constant (Kp and Kc) and Reaction quotient
The nomenclature for Equilibrium Constant (Kp and Kc) and Reaction Quotient is based on the following conventions:
- Equilibrium Constant: a. The symbol for Equilibrium Constant with respect to pressure is Kp, where the “p” stands for partial pressure. b. The symbol for Equilibrium Constant with respect to concentration is Kc, where the “c” stands for concentration. c. The equilibrium constant is written with the symbol K followed by a subscript that indicates the specific type of equilibrium constant, such as Kp or Kc. d. The numerical value of the equilibrium constant is written after the symbol, and it is unitless.
- Reaction Quotient: a. The symbol for Reaction Quotient with respect to pressure is Qp, where the “p” stands for partial pressure. b. The symbol for Reaction Quotient with respect to concentration is Qc, where the “c” stands for concentration. c. The reaction quotient is written with the symbol Q followed by a subscript that indicates the specific type of reaction quotient, such as Qp or Qc. d. The numerical value of the reaction quotient is written after the symbol, and it has the same units as the equilibrium constant.
In both cases, the coefficients of the reactants and products in the balanced chemical equation are used to calculate the value of Kp, Kc, Qp, or Qc. The nomenclature for Equilibrium Constant and Reaction Quotient is important because it helps to distinguish between the two concepts and to specify the type of equilibrium constant or reaction quotient being used in a particular calculation.
How is Required Equilibrium constant (Kp and Kc) and Reaction quotient
The Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are related to each other and are calculated using the same formula. The formula for calculating these values depends on the balanced chemical equation for the reaction under consideration.
The general formula for the equilibrium constant Kp, in terms of the partial pressures of the reactants and products, is:
Kp = (P_Products)^n / (P_Reactants)^m
where P_Products is the partial pressure of the products raised to the power of their stoichiometric coefficients (n), and P_Reactants is the partial pressure of the reactants raised to the power of their stoichiometric coefficients (m).
Similarly, the general formula for the equilibrium constant Kc, in terms of the molar concentrations of the reactants and products, is:
Kc = ([Products]^n) / ([Reactants]^m)
where [Products] is the molar concentration of the products raised to the power of their stoichiometric coefficients (n), and [Reactants] is the molar concentration of the reactants raised to the power of their stoichiometric coefficients (m).
The Reaction Quotient, denoted by Qp or Qc, is the same as the equilibrium constant formula, except it is calculated using the initial concentrations or partial pressures of the reactants and products, rather than their equilibrium concentrations or partial pressures. Thus, the Reaction Quotient can be thought of as a measure of how far a reaction has progressed towards equilibrium at a given point in time.
If the Reaction Quotient is less than the Equilibrium Constant (Q < K), the reaction will proceed in the forward direction to reach equilibrium. If Q is greater than K (Q > K), the reaction will proceed in the reverse direction until equilibrium is reached. If Q is equal to K (Q = K), the reaction is at equilibrium and there will be no net change in the concentrations or partial pressures of the reactants and products.
Overall, the Required Equilibrium Constant (Kp and Kc) and Reaction Quotient are important concepts that allow chemists to predict and understand the behavior of reversible reactions.
Case Study on Equilibrium constant (Kp and Kc) and Reaction quotient
Case Study: The Haber-Bosch Process
The Haber-Bosch process is an important industrial process that uses the equilibrium constant and reaction quotient concepts to optimize the production of ammonia, which is used in the production of fertilizers and other chemical products. The process involves the reaction of nitrogen gas and hydrogen gas to form ammonia gas, which is an exothermic reaction:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g) ∆H = -92.4 kJ/mol
The Haber-Bosch process operates at high temperature and pressure, typically around 450°C and 200 atm, to achieve a high yield of ammonia. The equilibrium constant for the reaction can be calculated using the formula for Kp or Kc, and it depends on the temperature and pressure of the reaction system. At 450°C and 200 atm, the equilibrium constant Kp for the Haber-Bosch process is approximately 5.0 x 10^3.
However, the Haber-Bosch process is not driven to completion due to thermodynamic limitations, and the yield of ammonia is often less than 20%. To increase the yield of ammonia, the Haber-Bosch process uses a recirculation process, in which the unreacted nitrogen and hydrogen gases are recycled back into the reactor. The recirculation process involves monitoring the reaction quotient, Qp or Qc, and adjusting the temperature and pressure of the system accordingly to shift the equilibrium towards the production of ammonia.
For example, if the reaction quotient Qp is less than the equilibrium constant Kp, this indicates that there are more reactants than products, and the reaction will proceed in the forward direction to produce more ammonia. To increase the yield of ammonia, the temperature and pressure can be increased to shift the equilibrium towards the products.
Conversely, if the reaction quotient Qp is greater than the equilibrium constant Kp, this indicates that there are more products than reactants, and the reaction will proceed in the reverse direction to consume some of the products and produce more reactants. To increase the yield of ammonia, the temperature and pressure can be decreased to shift the equilibrium towards the reactants.
By carefully monitoring and adjusting the temperature and pressure of the system, the Haber-Bosch process can achieve a high yield of ammonia and optimize the production of fertilizers and other chemical products.
In conclusion, the Haber-Bosch process is a case study that highlights the importance of the equilibrium constant and reaction quotient concepts in the design and optimization of industrial chemical processes. These concepts allow chemists to predict and understand the behavior of reversible reactions and to optimize the yield of desired products.
White paper on Equilibrium constant (Kp and Kc) and Reaction quotient
Introduction:
Chemical reactions can proceed in both the forward and reverse directions, and when the rates of these two opposing reactions become equal, the system reaches a state of equilibrium. The equilibrium constant (Kp or Kc) and reaction quotient (Qp or Qc) are important concepts used to describe the state of equilibrium of a chemical reaction.
Equilibrium Constant:
The equilibrium constant is a measure of the extent to which a reversible chemical reaction proceeds to reach equilibrium. It is defined as the ratio of the product of the equilibrium concentrations or partial pressures of the products to the product of the equilibrium concentrations or partial pressures of the reactants, each raised to the power of their stoichiometric coefficient.
For example, the equilibrium constant Kp for the reaction:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
is given by the expression:
Kp = [NH3]^2 / ([N2] * [H2]^3)
where [N2], [H2], and [NH3] are the equilibrium partial pressures of nitrogen, hydrogen, and ammonia, respectively.
The equilibrium constant is dependent on temperature and pressure, and its value can be used to predict the direction in which the reaction will proceed to reach equilibrium. If the value of Kp is greater than 1, the reaction will proceed in the forward direction to reach equilibrium with more products. Conversely, if the value of Kp is less than 1, the reaction will proceed in the reverse direction to reach equilibrium with more reactants.
Reaction Quotient:
The reaction quotient is a measure of the relative concentrations or partial pressures of the products and reactants of a chemical reaction at a given point in time. It is calculated using the same formula as the equilibrium constant, but using the actual concentrations or partial pressures of the reactants and products at a given point in time instead of the equilibrium concentrations or partial pressures.
For example, the reaction quotient Qp for the reaction:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
at a given point in time is given by the expression:
Qp = [NH3]^2 / ([N2] * [H2]^3)
The value of the reaction quotient can be used to predict the direction in which the reaction will proceed to reach equilibrium. If the value of Qp is less than the equilibrium constant Kp, the reaction will proceed in the forward direction to reach equilibrium with more products. Conversely, if the value of Qp is greater than Kp, the reaction will proceed in the reverse direction to reach equilibrium with more reactants.
Applications:
The equilibrium constant and reaction quotient concepts have many applications in chemistry and chemical engineering. They are used to predict the extent of reaction, to optimize reaction conditions, and to design and control chemical processes. For example, the Haber-Bosch process, which is used to produce ammonia from nitrogen and hydrogen gases, relies on the equilibrium constant and reaction quotient to optimize the yield of ammonia and to recycle unreacted gases back into the reactor.
Conclusion:
In conclusion, the equilibrium constant and reaction quotient are important concepts that allow chemists and chemical engineers to understand and predict the behavior of reversible chemical reactions. These concepts are used to optimize reaction conditions and to design and control chemical processes. A thorough understanding of the equilibrium constant and reaction quotient concepts is essential for anyone working in the field of chemistry or chemical engineering.