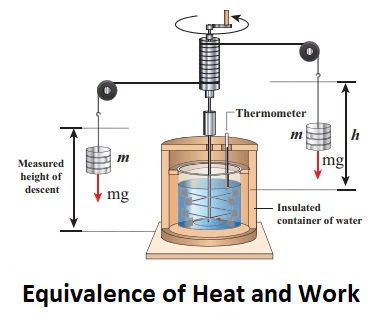
The equivalence of heat and work is known as the first law of thermodynamics, which states that energy cannot be created or destroyed, but it can be transformed from one form to another. Heat and work are two forms of energy that can be converted into each other.
When a system undergoes a change, the energy of the system changes, and this change can be accounted for by the heat transferred to or from the system or by the work done on or by the system. Heat and work are related by the equation:
Q = W + ΔU
where Q is the heat transferred to or from the system, W is the work done on or by the system, and ΔU is the change in internal energy of the system.
This equation states that the heat transferred to or from the system is equal to the work done on or by the system plus the change in internal energy of the system. This equation is a statement of the conservation of energy, which means that the total energy of a system is always conserved.
In summary, the first law of thermodynamics states that the heat transferred to or from a system is equal to the work done on or by the system plus the change in internal energy of the system. Therefore, heat and work are equivalent forms of energy that can be converted into each other.
What is Required Equivalence of Heat and Work
The required equivalence of heat and work refers to the concept that a given amount of heat and a given amount of work can produce the same change in the internal energy of a system, under certain conditions.
The equivalence between heat and work is based on the first law of thermodynamics, which states that the total energy of a closed system is constant. According to this law, the amount of energy transferred to or from a system can be expressed as the sum of the work done on the system and the heat transferred to the system.
If a system undergoes a change in its internal energy, this change can be brought about by either the transfer of heat or the performance of work on the system. In other words, the amount of energy required to produce a certain change in the internal energy of a system can be supplied by either heat or work, or a combination of both.
For example, suppose a gas is compressed from a certain volume to a smaller volume. This compression could be accomplished by the transfer of heat to the gas or by doing work on the gas, or by a combination of both. The amount of work done on the gas would depend on the force applied and the distance through which the gas is compressed. The amount of heat transferred to the gas would depend on the temperature difference between the gas and its surroundings.
Therefore, the required equivalence of heat and work means that if the same amount of energy is transferred to a system as heat or as work, and if all other conditions are the same, then the resulting change in the internal energy of the system will also be the same.
When is Required Equivalence of Heat and Work
The required equivalence of heat and work applies to any situation where a change in the internal energy of a system can be produced by either the transfer of heat or the performance of work on the system. This means that whenever there is a transfer of energy into or out of a system, the resulting change in the internal energy of the system can be achieved through a combination of heat and work.
The required equivalence of heat and work is a fundamental principle of thermodynamics and is based on the first law of thermodynamics, which states that energy cannot be created or destroyed, but it can be converted from one form to another. Therefore, the energy transferred to or from a system can be expressed as the sum of the work done on the system and the heat transferred to the system.
The required equivalence of heat and work applies to a wide range of systems and processes, from simple heat engines to complex industrial processes. For example, in an internal combustion engine, the energy produced by burning fuel is converted into work, which is used to power the vehicle. In a refrigerator, the transfer of heat from the interior of the refrigerator to the outside is achieved through the use of work, in the form of a compressor.
Overall, the required equivalence of heat and work is a fundamental concept in thermodynamics, and it plays a crucial role in understanding how energy is transferred and transformed in different systems and processes.
Where is Required Equivalence of Heat and Work
The required equivalence of heat and work is a fundamental principle of thermodynamics, which applies to a wide range of systems and processes. It can be observed in many different physical systems and phenomena, from simple heat engines to complex industrial processes.
In a heat engine, for example, heat is converted into work, and the efficiency of the engine is determined by the amount of heat that is converted into useful work. The required equivalence of heat and work is a crucial principle that helps to explain how this conversion process works and how it can be optimized.
In an industrial process, such as the production of electricity in a power plant, heat is often used to produce steam, which drives a turbine and generates electricity. The efficiency of the process depends on the amount of heat that is converted into work and the amount of heat that is lost to the environment. Again, the required equivalence of heat and work is a key principle that helps to explain how this process works and how it can be optimized.
The required equivalence of heat and work also plays a role in other fields of science and engineering, such as chemistry, materials science, and environmental science. In these fields, the principle helps to explain how energy is transferred and transformed in different chemical reactions and processes, and how this energy can be harnessed or controlled for specific purposes.
Overall, the required equivalence of heat and work is a fundamental principle that is observed in many different systems and processes, and it plays a crucial role in understanding the behavior of these systems and processes.
How is Required Equivalence of Heat and Work
The required equivalence of heat and work is based on the first law of thermodynamics, which states that the total energy of a closed system is constant. According to this law, the amount of energy transferred to or from a system can be expressed as the sum of the work done on the system and the heat transferred to the system. This means that the energy required to produce a certain change in the internal energy of a system can be supplied by either heat or work, or a combination of both.
The equivalence between heat and work is demonstrated by the work-energy theorem, which states that the work done on a system is equal to the change in the system’s kinetic energy. This theorem applies to both mechanical work and heat transfer, meaning that the energy transferred to a system as heat can also be expressed as the work required to produce the same change in the system’s kinetic energy.
For example, consider a gas that is compressed from a certain volume to a smaller volume. This compression could be accomplished by the transfer of heat to the gas or by doing work on the gas, or by a combination of both. The amount of work done on the gas would depend on the force applied and the distance through which the gas is compressed. The amount of heat transferred to the gas would depend on the temperature difference between the gas and its surroundings. However, regardless of whether the compression is accomplished by heat transfer or work, the resulting change in the internal energy of the gas would be the same.
Therefore, the required equivalence of heat and work is a fundamental principle of thermodynamics, which is based on the conservation of energy and the work-energy theorem. It applies to any situation where a change in the internal energy of a system can be produced by either the transfer of heat or the performance of work on the system.
Production of Equivalence of Heat and Work
The production of equivalence of heat and work is a fundamental aspect of many energy conversion processes, such as heat engines, refrigerators, and power plants. In these processes, the transfer of energy between the system and its surroundings can be achieved through a combination of heat and work, and the efficiency of the process depends on the ability to convert heat into work or vice versa.
One way to produce equivalence of heat and work is through the use of a heat engine. A heat engine is a device that converts thermal energy into mechanical work, and it operates by taking in heat from a high-temperature source, converting some of that heat into work, and releasing the remaining heat to a low-temperature sink. The efficiency of a heat engine is determined by the ratio of the work output to the heat input, and it depends on factors such as the temperature difference between the source and the sink and the design of the engine.
Another way to produce equivalence of heat and work is through the use of a refrigeration cycle. A refrigeration cycle is a process that uses work to transfer heat from a low-temperature environment to a high-temperature environment. In a typical refrigeration cycle, a working fluid is compressed to a high pressure, which raises its temperature above that of the surroundings. The fluid is then allowed to expand through a heat exchanger, which transfers heat from the low-temperature environment to the fluid. Finally, the fluid is compressed again and the cycle is repeated.
In a power plant, equivalence of heat and work can be produced by the use of a steam turbine. The steam turbine converts thermal energy from the combustion of fuel into mechanical work, which is used to generate electricity. The efficiency of the power plant depends on factors such as the temperature and pressure of the steam, the design of the turbine, and the efficiency of the generator.
Overall, the production of equivalence of heat and work is a fundamental aspect of many energy conversion processes, and it plays a crucial role in the efficient use of energy resources. By understanding the principles of thermodynamics and the ways in which energy can be converted from one form to another, engineers and scientists can develop more efficient and sustainable energy technologies for the future.
Case Study on Equivalence of Heat and Work
One example of the equivalence of heat and work is the operation of a steam turbine power plant. In this case study, we will explore how the principles of thermodynamics are applied to produce electricity through the conversion of thermal energy into mechanical work.
A steam turbine power plant operates by using the heat generated by the combustion of fuel, such as coal or natural gas, to produce steam. This steam is then used to drive a turbine, which in turn drives a generator to produce electricity.
The process begins with the combustion of fuel in a boiler, which generates heat that is transferred to water to produce steam. The steam is then directed into the turbine, where it expands and causes the blades of the turbine to rotate. As the turbine rotates, it drives a generator, which converts the mechanical energy into electrical energy.
The steam leaving the turbine is then condensed back into liquid form and returned to the boiler to be heated and transformed into steam once again. This cycle continues, with the same water being used repeatedly.
The efficiency of the power plant is determined by the ability to convert thermal energy into mechanical work. This conversion is achieved through the use of a Rankine cycle, which is a thermodynamic cycle that describes the operation of a steam turbine power plant.
In the Rankine cycle, the steam entering the turbine is typically at high pressure and high temperature. As the steam expands through the turbine, its pressure and temperature decrease, and some of its thermal energy is converted into mechanical work. The remaining thermal energy is then transferred to a condenser, where the steam is cooled and returned to liquid form.
The efficiency of the Rankine cycle is determined by the temperature difference between the high-temperature steam entering the turbine and the low-temperature steam leaving the condenser. The higher the temperature difference, the more efficient the cycle will be.
Overall, the operation of a steam turbine power plant demonstrates the equivalence of heat and work in action. The heat generated by the combustion of fuel is used to produce steam, which is then used to drive a turbine and produce mechanical work. Through this process, thermal energy is converted into electrical energy, demonstrating the fundamental principles of thermodynamics and the importance of the production of equivalence of heat and work in energy conversion processes.
White paper on Equivalence of Heat and Work
Introduction:
The equivalence of heat and work is a fundamental concept in thermodynamics, which describes the relationship between the transfer of heat and the performance of mechanical work. This concept has significant implications for energy conversion processes, such as heat engines, refrigerators, and power plants, where the transfer of energy between the system and its surroundings can be achieved through a combination of heat and work. This white paper aims to provide an overview of the concept of equivalence of heat and work, its applications in various energy conversion processes, and its significance in the field of thermodynamics.
Concept of Equivalence of Heat and Work:
The concept of equivalence of heat and work is based on the first law of thermodynamics, which states that energy cannot be created or destroyed but can be converted from one form to another. This law is often expressed as the equation:
ΔU = Q – W
where ΔU is the change in the internal energy of the system, Q is the heat transferred to the system, and W is the work done by the system. The equation states that the change in the internal energy of the system is equal to the difference between the heat transferred to the system and the work done by the system.
This equation demonstrates the equivalence of heat and work, as both are forms of energy that can be used to change the internal energy of the system. In a heat engine, for example, heat is converted into mechanical work, while in a refrigerator, mechanical work is used to transfer heat from a low-temperature environment to a high-temperature environment.
Applications of Equivalence of Heat and Work:
The equivalence of heat and work has significant applications in various energy conversion processes, including:
- Heat Engines: A heat engine is a device that converts thermal energy into mechanical work. In a heat engine, heat is transferred from a high-temperature source to a low-temperature sink, and some of this heat is converted into mechanical work. The efficiency of a heat engine is determined by the ratio of the work output to the heat input.
- Refrigerators: A refrigerator is a device that uses mechanical work to transfer heat from a low-temperature environment to a high-temperature environment. In a refrigerator, a working fluid is compressed to a high pressure, which raises its temperature above that of the surroundings. The fluid is then allowed to expand through a heat exchanger, which transfers heat from the low-temperature environment to the fluid. Finally, the fluid is compressed again, and the cycle is repeated.
- Power Plants: Power plants use a variety of energy sources, such as coal, natural gas, or nuclear power, to generate electricity. In a steam turbine power plant, for example, heat is generated by the combustion of fuel in a boiler, which produces steam. The steam is then used to drive a turbine, which in turn drives a generator to produce electricity.
Significance of Equivalence of Heat and Work:
The equivalence of heat and work is a crucial concept in the field of thermodynamics, as it provides a fundamental understanding of how energy can be converted from one form to another. By understanding the principles of thermodynamics, engineers and scientists can develop more efficient and sustainable energy technologies for the future.
For example, the equivalence of heat and work is a key factor in the design of more efficient heat engines, which can help to reduce the use of fossil fuels and the associated environmental impacts. Similarly, the use of refrigeration cycles can help to reduce energy consumption and greenhouse gas emissions in the cooling of buildings and other applications.
Conclusion:
In conclusion, the equivalence of heat and work is a fundamental concept in thermodynamics that provides a fundamental understanding of how energy can be converted from one form to another. This concept is based on the first law of thermodynamics, which states that energy cannot be created or destroyed but can be converted from one form to another. The equivalence of heat and work has significant applications in various energy conversion processes, including heat engines, refrigerators, and power plants. By understanding the principles of thermodynamics and the equivalence of heat and work, engineers and scientists can develop more efficient and sustainable energy technologies for the future, which can help to reduce the use of fossil fuels and the associated environmental impacts.
