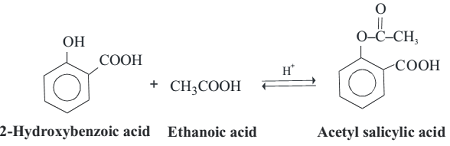
Phenols can undergo esterification reactions with carboxylic acids or acid chlorides to form esters. This reaction is typically catalyzed by an acid catalyst, such as sulfuric acid or hydrochloric acid, and can be carried out under reflux conditions.
The general reaction equation for the esterification of a phenol with a carboxylic acid is:
Ph-OH + RCOOH → Ph-OCOR + H2O
where Ph is a phenyl group, R is an alkyl or aryl group, and -OCOR is the ester linkage.
The reaction mechanism involves the protonation of the phenol oxygen by the acid catalyst, followed by the nucleophilic attack of the carboxylic acid on the electrophilic carbon of the phenol. The resulting intermediate then undergoes dehydration to form the ester product.
It should be noted that esterification of phenols can also occur through the reaction of the phenol with acid chlorides or anhydrides. Additionally, the presence of electron-donating or electron-withdrawing substituents on the phenol ring can affect the reactivity of the phenol and the selectivity of the esterification reaction.
What is Required Phenols Esterification
In order to carry out the esterification of phenols, the following reagents and conditions are typically required:
- Phenol: A phenol compound is required as the starting material for the reaction.
- Carboxylic acid: A carboxylic acid is required to react with the phenol to form the ester product.
- Acid catalyst: An acid catalyst, such as sulfuric acid or hydrochloric acid, is typically used to promote the reaction by protonating the phenol oxygen and activating the carboxylic acid.
- Solvent: A suitable solvent, such as toluene or dichloromethane, may be required to dissolve the reactants and facilitate the reaction.
- Reflux conditions: The reaction is typically carried out under reflux conditions, which involves heating the reaction mixture to boiling and then cooling the vapor that forms back into the reaction vessel. This helps to ensure that the reaction proceeds to completion and that the ester product is formed in good yield.
- Workup reagents: After the reaction is complete, the ester product may need to be purified and isolated from the reaction mixture. This can be done using workup reagents such as aqueous sodium bicarbonate to neutralize the acid catalyst and extract the ester product, or by distillation to separate the ester product from the solvent and any remaining reactants.
When is Required Phenols Esterification
Phenols esterification is a versatile reaction that can be used in a variety of contexts, including:
- Synthesis of esters: Phenols esterification is commonly used in the synthesis of esters, which are important compounds with a wide range of applications. For example, esters are used as fragrances, flavorings, solvents, and plasticizers, among other things.
- Medicinal chemistry: Phenols esterification can be used in the synthesis of bioactive compounds for use in medicinal chemistry. For example, aspirin is synthesized by esterification of salicylic acid with acetic anhydride.
- Polymer chemistry: Phenols esterification can be used in the synthesis of polymers and other materials. For example, polycarbonate resins can be synthesized by esterification of bisphenol A with phosgene.
- Natural product synthesis: Phenols esterification can be used in the synthesis of natural products, which are important sources of medicines and other biologically active compounds. For example, esters of salicylic acid are found in natural products such as wintergreen oil.
Overall, phenols esterification is a versatile reaction that can be used in a wide range of contexts for the synthesis of important compounds.
Where is Required Phenols Esterification
Phenols esterification can be used in a variety of industries and applications, including:
- Pharmaceutical industry: Phenols esterification is widely used in the synthesis of drugs and other biologically active compounds, including aspirin, penicillin, and steroids.
- Fragrance and flavor industry: Phenols esterification is used in the synthesis of fragrances and flavorings for use in perfumes, cosmetics, and food products.
- Polymer industry: Phenols esterification is used in the synthesis of polymers and other materials, including polycarbonates, polyester resins, and polyurethanes.
- Agrochemical industry: Phenols esterification is used in the synthesis of herbicides, insecticides, and other agrochemicals.
- Research and development: Phenols esterification is a common reaction in research and development laboratories for the synthesis of new compounds and materials.
Overall, phenols esterification is an important reaction in a wide range of industries and applications, due to its versatility and ability to produce valuable compounds.
How is Required Phenols Esterification
The process of phenols esterification typically involves the following steps:
- Preparation of the reactants: The phenol and carboxylic acid reactants are typically prepared by weighing out the appropriate amounts and dissolving them in a suitable solvent, such as toluene or dichloromethane.
- Addition of acid catalyst: The acid catalyst, such as sulfuric acid or hydrochloric acid, is then added to the reaction mixture to promote the reaction by protonating the phenol oxygen and activating the carboxylic acid.
- Heating under reflux: The reaction mixture is heated under reflux conditions, typically using a condenser to prevent the loss of volatile components, for several hours to promote the esterification reaction.
- Workup and isolation of the product: Once the reaction is complete, the ester product is typically isolated and purified from the reaction mixture using workup reagents such as aqueous sodium bicarbonate or by distillation.
The conditions and reaction times for phenols esterification may vary depending on the specific reactants and reaction conditions used, as well as the desired yield and purity of the ester product. The reaction can be monitored by techniques such as thin-layer chromatography (TLC) or gas chromatography (GC) to determine the extent of the reaction and the purity of the product.
Production of Phenols Esterification
The production of phenols esterification can be carried out at various scales, ranging from small laboratory-scale reactions to large-scale industrial processes. The production process typically involves the following steps:
- Raw materials preparation: The raw materials, including the phenol and carboxylic acid reactants, as well as the acid catalyst, are prepared in large quantities according to the desired reaction conditions.
- Reactor setup: The reactants are then combined in a reactor vessel, which is equipped with a heating and cooling system, stirrer, and reflux condenser. The reactor vessel may also be equipped with additional sensors and control systems for monitoring and controlling the reaction conditions.
- Reaction: The reaction is then carried out under reflux conditions, typically using a large amount of solvent to dissolve the reactants and facilitate the reaction. The reaction temperature, pressure, and duration are carefully controlled to ensure optimal yields and purity of the ester product.
- Workup and purification: Once the reaction is complete, the ester product is typically isolated and purified from the reaction mixture using workup reagents such as aqueous sodium bicarbonate or by distillation. The purified product is then packaged and shipped to customers or used as a starting material for further chemical synthesis.
The scale and complexity of the production process may vary depending on the specific application and requirements of the product. For example, the production of pharmaceutical-grade esters may require additional purification steps and stringent quality control measures to ensure compliance with regulatory standards.
Case Study on Phenols Esterification
One example of a case study on phenols esterification involves the synthesis of a bioactive compound called 4-(4-hydroxyphenyl)butan-2-one, which has potential anti-inflammatory and anti-tumor properties.
The synthesis of this compound can be achieved through phenols esterification of 4-hydroxybenzoic acid with 4-bromobutan-2-one. The reaction is typically carried out in the presence of a strong acid catalyst, such as sulfuric acid, and a large amount of solvent, such as toluene or dichloromethane.
In a study published in the journal Bioorganic & Medicinal Chemistry Letters, researchers synthesized 4-(4-hydroxyphenyl)butan-2-one using phenols esterification and evaluated its anti-inflammatory and anti-tumor activities. They found that the compound exhibited potent anti-inflammatory activity, as evidenced by its ability to inhibit the production of pro-inflammatory cytokines in a mouse model of inflammation. The compound also showed promise as an anti-tumor agent, as it inhibited the growth of human cancer cell lines in vitro.
The study highlights the versatility of phenols esterification in the synthesis of bioactive compounds with potential pharmaceutical applications. By carefully controlling the reaction conditions, researchers can produce compounds with specific properties and activities that may have therapeutic value.
White paper on Phenols Esterification
Introduction
Phenols esterification is a widely used reaction in the synthesis of various compounds and materials across different industries. The reaction involves the reaction of a phenol and a carboxylic acid in the presence of an acid catalyst to form an ester. This white paper aims to provide an overview of phenols esterification, including its mechanism, applications, and benefits.
Mechanism of Phenols Esterification
Phenols esterification involves the reaction of a phenol and a carboxylic acid in the presence of an acid catalyst. The reaction proceeds through an electrophilic aromatic substitution mechanism, in which the acid catalyst protonates the phenol oxygen to form a more reactive electrophile. The carboxylic acid then attacks the electrophilic carbon atom of the phenol, leading to the formation of an intermediate product. The intermediate product is then deprotonated by the acid catalyst to yield the final ester product.
Applications of Phenols Esterification
Phenols esterification is a versatile reaction that is used in various industries and applications. Some of the common applications of phenols esterification include:
- Pharmaceutical industry: Phenols esterification is widely used in the synthesis of drugs and other biologically active compounds, including aspirin, penicillin, and steroids.
- Fragrance and flavor industry: Phenols esterification is used in the synthesis of fragrances and flavorings for use in perfumes, cosmetics, and food products.
- Polymer industry: Phenols esterification is used in the synthesis of polymers and other materials, including polycarbonates, polyester resins, and polyurethanes.
- Agrochemical industry: Phenols esterification is used in the synthesis of herbicides, insecticides, and other agrochemicals.
Benefits of Phenols Esterification
Phenols esterification offers several benefits, including:
- Versatility: Phenols esterification can be used to produce a wide range of compounds with different properties and activities.
- Efficiency: Phenols esterification is a relatively simple and efficient reaction that can be carried out under mild conditions and with relatively low energy inputs.
- Scalability: Phenols esterification can be scaled up to industrial levels, making it a useful reaction for large-scale production of chemicals and materials.
- Selectivity: Phenols esterification can be carefully controlled to produce specific products with high selectivity and purity.
Conclusion
Phenols esterification is an important reaction in the synthesis of various compounds and materials across different industries. The reaction offers several benefits, including versatility, efficiency, scalability, and selectivity. As such, it is an essential tool for chemical synthesis and manufacturing, with potential applications in areas such as pharmaceuticals, fragrances, polymers, and agrochemicals.
