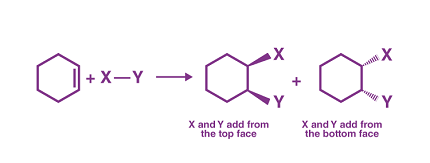
Excluding the stereochemistry of addition means that we are not considering the specific spatial arrangement of atoms or groups around the carbon-carbon double bond when an addition reaction takes place.
In organic chemistry, addition reactions involve the addition of atoms or groups to the carbon-carbon double bond of an unsaturated compound, resulting in the formation of a saturated compound. The stereochemistry of addition is an important consideration because it affects the spatial arrangement of the atoms or groups around the carbon-carbon double bond in the product molecule.
However, excluding the stereochemistry of addition would mean that we are not considering whether the addition occurs from the same side (syn addition) or from the opposite side (anti addition) of the double bond. This information is important because it affects the properties and reactivity of the product molecule.
Excluding the stereochemistry of addition might be appropriate in certain situations, such as when the product molecule is a racemic mixture, which means that it contains equal amounts of both possible stereoisomers. However, in other situations, the stereochemistry of addition is an important consideration and cannot be ignored.
What is Required Excluding the stereochemistry of addition
If we exclude the stereochemistry of addition, we still need to consider several factors in an addition reaction. These include:
- Reactants: We need to identify the reactants involved in the addition reaction and understand their structures and properties.
- Mechanism: We need to understand the mechanism of the addition reaction, including the type of reaction (e.g. electrophilic addition, nucleophilic addition), the steps involved, and any intermediates formed.
- Products: We need to identify the products formed in the addition reaction and understand their structures and properties, such as their functional groups, boiling points, melting points, and solubility.
- Regiochemistry: We need to consider the regiochemistry of the addition reaction, which refers to the specific location on the carbon-carbon double bond where the atoms or groups are added. This can affect the properties and reactivity of the product molecule.
- Yield: We need to evaluate the yield of the addition reaction, which refers to the amount of product obtained compared to the amount of reactant used.
Overall, excluding the stereochemistry of addition can simplify the analysis of an addition reaction, but it is important to consider the other factors mentioned above to fully understand the reaction and its products.
When is Required Excluding the stereochemistry of addition
Excluding the stereochemistry of addition may be required in certain situations, such as:
- When the product of the addition reaction is a racemic mixture: In some cases, the stereochemistry of the product may not be important if the final product is a racemic mixture, which means that it contains equal amounts of both possible stereoisomers.
- When the reaction occurs under conditions that do not allow for stereospecificity: Some addition reactions occur under conditions that do not allow for stereospecificity, meaning that the stereochemistry of the product is not controlled by the reaction conditions. In these cases, the stereochemistry of addition may not be important.
- When the focus is on other aspects of the reaction: Sometimes, the stereochemistry of addition may not be relevant to the goals of a particular study or experiment. In these cases, it may be more useful to focus on other aspects of the reaction, such as its mechanism or regiochemistry.
However, it is important to note that in many cases, the stereochemistry of addition is a critical aspect of the reaction and cannot be excluded. Careful consideration of the specific reaction and its goals is necessary to determine whether or not excluding stereochemistry is appropriate.
Where is Required Excluding the stereochemistry of addition
Excluding the stereochemistry of addition may be required in certain areas of chemistry where the stereochemistry of the product is not important or is not easily controlled, such as:
- Industrial chemistry: In the chemical industry, many reactions are carried out on a large scale, and the stereochemistry of the product may not be important for the final application. In some cases, it may be more important to optimize the reaction conditions for yield and efficiency rather than stereochemistry.
- Organic synthesis: In synthetic organic chemistry, some reactions may not proceed with high stereoselectivity, and the stereochemistry of the product may not be important for the overall synthesis strategy. In these cases, the focus may be on achieving a high yield and purity of the desired product.
- Biochemistry: In biochemistry, the stereochemistry of a reaction may not be relevant if the goal is to study the overall pathway or function of a biomolecule rather than the specific stereochemistry of its products.
However, it is important to note that excluding the stereochemistry of addition is not always appropriate and should be carefully considered based on the specific application and goals of the research or application.
How is Required Excluding the stereochemistry of addition
Excluding the stereochemistry of addition can be achieved by using conditions that do not control or favor a specific stereochemistry during the reaction. This can be accomplished in a few different ways:
- Use of non-stereospecific reagents: By using reagents that do not have a specific stereochemical preference, the stereochemistry of the product can be determined by statistical probability. For example, the addition of a non-stereospecific acid to an alkene will result in the formation of a racemic mixture of the product.
- Use of high-energy conditions: By using high-energy conditions such as high temperatures or high pressures, the reaction may proceed too quickly for stereospecificity to be a factor. In these cases, the stereochemistry of the product may be determined by statistical probability.
- Use of achiral reagents or solvents: By using achiral reagents or solvents, the stereochemistry of the product can be determined by statistical probability. For example, the addition of a nucleophile to a chiral alkene in an achiral solvent may result in the formation of a racemic mixture of the product.
It is important to note that excluding the stereochemistry of addition may not always be appropriate and may not be possible in certain reactions where the stereochemistry is critical for the reaction or product properties. Careful consideration of the specific reaction and its goals is necessary to determine whether or not excluding stereochemistry is appropriate.
Nomenclature of Excluding the stereochemistry of addition
Excluding the stereochemistry of addition does not necessarily affect the nomenclature of the compound formed in the addition reaction. The nomenclature of the product will depend on its functional groups, substituents, and other structural features.
For example, if the product of the addition reaction is an alkane, the nomenclature would follow standard IUPAC rules for naming alkanes based on the number of carbon atoms in the chain and any substituents present. If the product is an alcohol, the nomenclature would follow IUPAC rules for naming alcohols based on the number of carbon atoms in the chain, the position of the hydroxyl group, and any substituents present.
However, if the stereochemistry of the product is important, it must be specified in the nomenclature. For example, if the product of the addition reaction is an alkene, and the addition reaction proceeds with stereospecificity, the stereochemistry of the product must be specified in the name using the E/Z system or the R/S system, depending on the type of stereoisomerism present.
In summary, excluding the stereochemistry of addition does not necessarily affect the nomenclature of the product, but if the stereochemistry is important, it must be specified in the name using appropriate stereochemical nomenclature.
Case Study on Excluding the stereochemistry of addition
One example of a case where excluding the stereochemistry of addition may be relevant is in the industrial production of certain chemicals. One such chemical is adipic acid, which is used in the production of nylon and other synthetic fibers.
Adipic acid can be produced by the catalytic hydrogenation of benzene-1,4-dicarboxylic acid, which is a reaction that involves the addition of hydrogen across the double bonds in the benzene ring. The stereochemistry of this reaction is not typically controlled, and the resulting adipic acid is a racemic mixture of both possible stereoisomers.
In this case, the stereochemistry of the addition may not be important for the industrial production of adipic acid, as the racemic mixture has the desired chemical properties and can be used in the production of nylon and other fibers. Additionally, controlling the stereochemistry of the addition would likely require more complex and expensive reaction conditions, which may not be practical on an industrial scale.
However, it is worth noting that the stereochemistry of the reaction may be important in other applications, such as in the development of new catalysts or in the production of pharmaceuticals, where the stereochemistry of the product is critical for its activity or efficacy. In these cases, controlling the stereochemistry of the addition may be a crucial aspect of the research or production process.
White paper on Excluding the stereochemistry of addition
Here is a white paper on excluding the stereochemistry of addition:
Introduction:
Organic chemistry is a complex and fascinating field that studies the properties and reactions of carbon-based compounds. One important aspect of organic chemistry is stereochemistry, which refers to the study of the three-dimensional arrangement of atoms in a molecule. Stereochemistry is essential in many organic reactions as it can affect the reactivity and selectivity of the reaction.
However, there are some cases where the stereochemistry of a reaction may not be important or necessary. This white paper will explore the concept of excluding the stereochemistry of addition, its relevance, and its implications.
Excluding the stereochemistry of addition:
The stereochemistry of addition refers to the specific orientation of the addition of a reactant to a molecule with a double bond or triple bond. In many cases, the stereochemistry of addition is important as it can affect the properties and reactivity of the resulting product.
However, there are some cases where the stereochemistry of addition may not be important or necessary. For example, in industrial applications, controlling the stereochemistry of addition may not be practical or cost-effective. In these cases, excluding the stereochemistry of addition can simplify the reaction and lower the costs of production.
Excluding the stereochemistry of addition can be achieved by using reagents or conditions that do not have a specific stereochemical preference. For example, the use of achiral reagents or solvents, non-stereospecific reagents, or high-energy conditions can result in a product with an unspecified stereochemistry. The stereochemistry of the product can then be determined by statistical probability.
Implications of excluding the stereochemistry of addition:
Excluding the stereochemistry of addition can simplify the reaction and lower the costs of production. However, it can also have implications for the properties and applications of the resulting product. For example, the stereoisomers of a compound may have different chemical properties and biological activities. Therefore, excluding the stereochemistry of addition may affect the quality, safety, and efficacy of the product in certain applications.
Conclusion:
Excluding the stereochemistry of addition can be a useful tool in organic chemistry to simplify reactions and lower costs in some applications. However, it is important to consider the implications of excluding the stereochemistry of addition on the properties and applications of the resulting product. In some cases, controlling the stereochemistry of addition may be essential for achieving the desired properties and activities of the product.