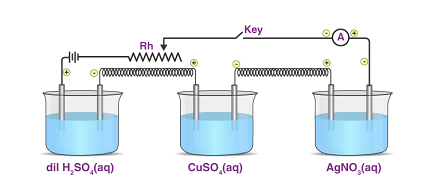
Faraday’s laws of electrolysis are two fundamental laws that describe the quantitative relationship between the amount of electric charge passed through an electrolytic cell and the amount of chemical change that occurs during electrolysis. These laws were developed by the British scientist Michael Faraday in the early 19th century.
Faraday’s First Law of Electrolysis states that the amount of a substance produced or consumed during electrolysis is directly proportional to the amount of electric charge passed through the cell. In other words, the more electric charge that passes through the cell, the more chemical reaction takes place. Mathematically, this law can be expressed as:
m = Q / (nF)
where m is the mass of the substance produced or consumed, Q is the electric charge passed through the cell, n is the number of moles of electrons exchanged in the reaction, and F is the Faraday constant (the charge on one mole of electrons).
Faraday’s Second Law of Electrolysis states that the amount of different substances produced by the same amount of electric charge passing through the cell is proportional to their respective equivalent weights. An equivalent weight is the mass of a substance that will combine with or displace one mole of electrons during electrolysis. Mathematically, this law can be expressed as:
m₁ / m₂ = E₁ / E₂
where m₁ and m₂ are the masses of two different substances produced during electrolysis, and E₁ and E₂ are their respective equivalent weights.
These laws have important practical applications in electrochemistry, such as in the production of metals by electrolysis and in the measurement of the electrochemical properties of materials.
What is Required Faraday’s laws of electrolysis
To apply Faraday’s laws of electrolysis, the following conditions are required:
- The electrolytic cell must be a closed system, meaning that no substances can enter or leave the cell during electrolysis.
- The electrolyte must be a solution of ions that can be reduced or oxidized at the electrodes.
- A direct current (DC) power source must be used to supply a constant flow of electrons through the cell.
- The electrodes must be inert and not participate in the chemical reaction. Platinum or graphite electrodes are commonly used.
- The temperature, pressure, and other environmental factors must remain constant throughout the experiment.
By ensuring that these conditions are met, the quantitative relationship between the amount of electric charge passed through the cell and the amount of chemical reaction that occurs can be accurately determined using Faraday’s laws of electrolysis.
Who is Required Faraday’s laws of electrolysis
Faraday’s laws of electrolysis were discovered and developed by the British scientist Michael Faraday in the early 19th century. Faraday was a renowned physicist and chemist who made significant contributions to the fields of electromagnetism and electrochemistry. He is widely regarded as one of the greatest experimentalists in the history of science and is known for his pioneering work on the relationship between electricity and chemical reactions. Faraday’s laws of electrolysis remain an essential part of electrochemistry and are widely used today in various fields, including metallurgy, electroplating, and battery technology.
When is Required Faraday’s laws of electrolysis
Faraday’s laws of electrolysis are required whenever there is a need to quantify the amount of chemical reaction that occurs during electrolysis. Electrolysis is a process that uses an electric current to drive a non-spontaneous chemical reaction, and it has numerous practical applications in various fields, including metallurgy, electroplating, and battery technology.
For example, Faraday’s laws of electrolysis can be used to determine the amount of metal that can be produced by the electrolysis of a metal salt solution. They can also be used to calculate the efficiency of an electroplating process by measuring the amount of metal deposited on a substrate.
In general, whenever an electrochemical reaction is used to produce or consume a substance, Faraday’s laws can be used to determine the amount of material produced or consumed and to optimize the process parameters for better efficiency.
Where is Required Faraday’s laws of electrolysis
Faraday’s laws of electrolysis are required in various applications where electrolysis is used to produce or consume substances. Some common examples of where Faraday’s laws of electrolysis are applied include:
- Metallurgy: Faraday’s laws of electrolysis are used to determine the amount of metal that can be produced by the electrolysis of a metal salt solution. This information is used to optimize the production of metals such as aluminum, magnesium, and copper.
- Electroplating: Faraday’s laws of electrolysis are used to calculate the amount of metal that is deposited on a substrate during an electroplating process. This information is used to optimize the process parameters to achieve the desired coating thickness and quality.
- Batteries: Faraday’s laws of electrolysis are used to calculate the amount of charge that a battery can store and deliver. This information is used to optimize the design and performance of batteries in various applications, including electric vehicles and portable electronic devices.
- Chemical synthesis: Faraday’s laws of electrolysis are used to produce chemicals and drugs in large quantities. This information is used to optimize the process parameters to achieve high yields and purity.
In general, Faraday’s laws of electrolysis are required in any application where the production or consumption of substances through electrochemical reactions is necessary, including industrial processes, laboratory experiments, and research and development.
How is Required Faraday’s laws of electrolysis
Faraday’s laws of electrolysis are applied using mathematical equations that relate the amount of electric charge passed through an electrolytic cell to the amount of chemical reaction that occurs. There are two fundamental laws of electrolysis that were developed by Michael Faraday, as explained earlier:
- Faraday’s First Law of Electrolysis: This law states that the amount of a substance produced or consumed during electrolysis is directly proportional to the amount of electric charge passed through the cell. The equation for this law is:m = Q / (nF)
where m is the mass of the substance produced or consumed, Q is the electric charge passed through the cell, n is the number of moles of electrons exchanged in the reaction, and F is the Faraday constant.
- Faraday’s Second Law of Electrolysis: This law states that the amount of different substances produced by the same amount of electric charge passing through the cell is proportional to their respective equivalent weights. The equation for this law is:m₁ / m₂ = E₁ / E₂
where m₁ and m₂ are the masses of two different substances produced during electrolysis, and E₁ and E₂ are their respective equivalent weights.
To apply these laws, the experimental conditions must be carefully controlled and the necessary measurements made. The amount of electric charge passed through the cell can be measured using an ammeter, while the mass of the substances produced or consumed can be measured using a balance. Once the experimental data is collected, the equations can be used to calculate the desired quantities.
In summary, Faraday’s laws of electrolysis are applied using mathematical equations that relate the amount of electric charge passed through an electrolytic cell to the amount of chemical reaction that occurs. The laws are used in various applications, including metallurgy, electroplating, battery technology, and chemical synthesis.
Case Study on Faraday’s laws of electrolysis
One example of the application of Faraday’s laws of electrolysis is in the production of aluminum metal from its ore bauxite. This process involves the extraction of aluminum ions from a solution of aluminum oxide dissolved in molten cryolite using an electrolytic cell.
In this process, aluminum oxide (Al₂O₃) is dissolved in molten cryolite (Na₃AlF₆) to form a solution of aluminum ions (Al³⁺) and fluoride ions (F⁻). The solution is then electrolyzed using a carbon electrode as the cathode and a carbon electrode coated with molten cryolite as the anode. When an electric current is passed through the solution, aluminum ions are reduced to form aluminum metal at the cathode, while fluoride ions are oxidized to form fluorine gas at the anode.
To apply Faraday’s laws of electrolysis to this process, the amount of electric charge passed through the cell and the mass of aluminum produced must be measured. According to Faraday’s First Law of Electrolysis, the mass of aluminum produced is directly proportional to the amount of electric charge passed through the cell.
The equation for this law is:
m = Q / (nF)
where m is the mass of aluminum produced, Q is the electric charge passed through the cell, n is the number of moles of electrons exchanged in the reaction, and F is the Faraday constant.
The Faraday constant is a constant of proportionality that relates the amount of electric charge to the amount of substance produced or consumed. Its value is 96,485 coulombs per mole of electrons.
The number of moles of electrons exchanged in the reduction of aluminum ions to aluminum metal is three, since each aluminum ion gains three electrons to form an aluminum atom. Therefore, the equation for the mass of aluminum produced becomes:
m = Q / (3F)
To calculate the mass of aluminum produced, the amount of electric charge passed through the cell must be measured using an ammeter. Once this value is known, it can be substituted into the equation to obtain the mass of aluminum produced.
The production of aluminum by electrolysis of aluminum oxide is an important industrial process that makes use of Faraday’s laws of electrolysis. By carefully controlling the process parameters, such as the temperature and composition of the electrolytic solution and the current density, it is possible to achieve high yields of aluminum with high purity and efficiency.
White paper on Faraday’s laws of electrolysis
Introduction:
Faraday’s laws of electrolysis are fundamental laws that govern the relationship between the amount of electric charge passed through an electrolytic cell and the amount of chemical reaction that occurs. These laws were first described by the British scientist Michael Faraday in the early 19th century and are widely used in various fields, including metallurgy, electroplating, battery technology, and chemical synthesis.
In this white paper, we will discuss the basics of Faraday’s laws of electrolysis, their applications, and their relevance in modern science and technology.
Faraday’s First Law of Electrolysis:
Faraday’s First Law of Electrolysis states that the amount of a substance produced or consumed during electrolysis is directly proportional to the amount of electric charge passed through the cell. This law can be expressed mathematically using the following equation:
m = Q / (nF)
where m is the mass of the substance produced or consumed, Q is the electric charge passed through the cell, n is the number of moles of electrons exchanged in the reaction, and F is the Faraday constant.
The Faraday constant is a physical constant that relates the amount of electric charge to the amount of substance produced or consumed. Its value is 96,485 coulombs per mole of electrons.
Faraday’s Second Law of Electrolysis:
Faraday’s Second Law of Electrolysis states that the amount of different substances produced by the same amount of electric charge passing through the cell is proportional to their respective equivalent weights. This law can be expressed mathematically using the following equation:
m₁ / m₂ = E₁ / E₂
where m₁ and m₂ are the masses of two different substances produced during electrolysis, and E₁ and E₂ are their respective equivalent weights.
The equivalent weight of a substance is defined as the amount of the substance that reacts with one mole of electrons in an electrolytic cell. It is calculated by dividing the molar mass of the substance by the number of moles of electrons exchanged in the reaction.
Applications of Faraday’s Laws of Electrolysis:
Faraday’s laws of electrolysis have numerous applications in modern science and technology. One of the most important applications is in the production of metals from their ores by electrolysis. For example, the extraction of aluminum from its ore bauxite involves the electrolysis of a solution of aluminum oxide dissolved in molten cryolite using a carbon electrode as the cathode and a carbon electrode coated with molten cryolite as the anode.
Other applications of Faraday’s laws of electrolysis include electroplating, which involves the deposition of a thin layer of metal onto a substrate by electrolysis, and battery technology, which involves the conversion of chemical energy into electrical energy by means of an electrochemical reaction.
Relevance of Faraday’s Laws of Electrolysis in Modern Science and Technology:
Faraday’s laws of electrolysis remain highly relevant in modern science and technology, as they provide a fundamental understanding of the relationship between electricity and chemical reactions. This understanding is essential for the development of new materials, processes, and technologies, such as new battery technologies for energy storage, electrochemical sensors for environmental monitoring, and electroplating techniques for the production of advanced materials.
Conclusion:
Faraday’s laws of electrolysis are fundamental laws that govern the relationship between electricity and chemical reactions in electrolytic cells. These laws have numerous applications in modern science and technology, and their relevance continues to grow as new materials, processes, and technologies are developed. A thorough understanding of Faraday’s laws of electrolysis is essential for the design and optimization of electrolytic processes and the development of new electrochemical technologies.
