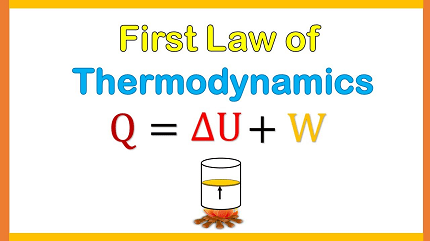
The First Law of Thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, but it can be transformed from one form to another or transferred from one system to another. In other words, the total energy of a closed system remains constant. This law is a fundamental principle of physics and has many applications in engineering, chemistry, and other fields. The first law can be expressed mathematically as:
ΔU = Q – W
where ΔU is the change in internal energy of a system, Q is the heat transferred to the system, and W is the work done by the system. This equation shows that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
What is Thermal Physics First law of thermodynamics
In thermal physics, the First Law of Thermodynamics is a fundamental principle that states that energy cannot be created or destroyed but can be converted from one form to another. This law is based on the principle of conservation of energy.
Thermal physics deals with the study of the behavior of matter at the microscopic level, particularly with regard to its thermal properties. The First Law of Thermodynamics applies to systems that involve thermal energy, such as gases, liquids and solids.
The law states that the change in the internal energy of a system is equal to the heat added to the system minus the work done by the system. This means that if heat is added to a system, the internal energy of the system will increase. If work is done on the system, its internal energy will also increase.
The First Law of Thermodynamics is important in understanding the behavior of many physical systems. For example, it is used to analyze the efficiency of heat engines, which convert thermal energy into mechanical energy. It is also used in the study of heat transfer, which is important in designing efficient heating and cooling systems.
Overall, the First Law of Thermodynamics is a fundamental principle of thermal physics that has many important practical applications. It forms the basis for the study of thermodynamics, which is concerned with the relationships between heat, work, and energy in systems.
When is Thermal Physics First law of thermodynamics
The First Law of Thermodynamics is applicable in thermal physics when dealing with systems that involve thermal energy. Thermal physics is the study of the behavior of matter at the microscopic level, particularly with regard to its thermal properties, such as temperature, heat, and entropy.
The First Law of Thermodynamics applies to systems where energy is transferred in the form of heat or work. Heat refers to the transfer of thermal energy between a system and its surroundings due to a temperature difference, while work refers to the transfer of energy due to a mechanical force acting on a system.
The law states that the change in the internal energy of a system is equal to the heat added to the system minus the work done by the system. This means that the internal energy of a system can be changed by adding or removing heat, or by doing work on or by the system.
Thermal physics is used in many practical applications, such as in the design of refrigeration and air conditioning systems, the study of the behavior of materials at high temperatures, and the analysis of the efficiency of heat engines.
Overall, the First Law of Thermodynamics is a fundamental principle of thermal physics and is applicable whenever energy is transferred as heat or work in a system.
Where is Thermal Physics First law of thermodynamics
The First Law of Thermodynamics is a fundamental principle of thermal physics that applies to systems that involve thermal energy. Thermal physics is the study of the behavior of matter at the microscopic level, particularly with regard to its thermal properties, such as temperature, heat, and entropy.
Thermal systems can be found in many different physical contexts, such as engines, refrigeration systems, combustion processes, and materials science. For example, thermal physics is used in the design of engines that convert thermal energy into mechanical energy, such as car engines and power plants. It is also used in the design of refrigeration and air conditioning systems, which transfer heat from one location to another.
In materials science, thermal physics is used to study the behavior of materials at high temperatures, such as the melting and solidification of metals. It is also used in the study of phase transitions, such as the transition from a liquid to a gas.
Overall, the First Law of Thermodynamics is applicable in any system that involves the transfer of thermal energy, and thermal physics is used in a wide range of scientific and engineering applications.
How is Thermal Physics First law of thermodynamics
The First Law of Thermodynamics in thermal physics is expressed mathematically as:
ΔU = Q – W
where ΔU is the change in internal energy of a system, Q is the heat added to the system, and W is the work done by the system. The law states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
This law is based on the principle of conservation of energy, which states that energy cannot be created or destroyed but can only be converted from one form to another. In the case of thermal systems, energy can be transferred as heat or work.
The law can be used to analyze and predict the behavior of thermal systems, such as heat engines and refrigeration systems. For example, the efficiency of a heat engine can be calculated using the First Law of Thermodynamics, which relates the work output of the engine to the heat input.
The law also has important implications for the study of thermodynamics, which is concerned with the relationships between heat, work, and energy in systems. The First Law of Thermodynamics is the basis for the study of thermodynamics and is fundamental to many areas of science and engineering, including materials science, chemistry, and mechanical engineering.
Overall, the First Law of Thermodynamics is a fundamental principle of thermal physics that describes the behavior of thermal systems and provides a framework for the study of thermodynamics.
Case Study on Thermal Physics First law of thermodynamics
One example of how the First Law of Thermodynamics is used in practice is in the design and operation of a steam turbine power plant.
In a steam turbine power plant, thermal energy is converted into mechanical energy to generate electricity. The process involves a number of steps, including the combustion of fuel to heat water in a boiler, the production of steam, the expansion of steam through a turbine, and the condensation of steam back to water for reuse.
The First Law of Thermodynamics is used to analyze and optimize the performance of the power plant. For example, the law can be used to calculate the efficiency of the power plant, which is defined as the ratio of the work output to the heat input.
The heat input to the power plant is the energy released by the combustion of fuel in the boiler, while the work output is the mechanical energy generated by the turbine. The difference between the heat input and the work output represents the heat rejected by the power plant, which is typically released to the environment through a cooling tower or other means.
By applying the First Law of Thermodynamics to the power plant, engineers can identify ways to increase the efficiency of the system, such as by improving the combustion process, increasing the steam temperature and pressure, or reducing losses due to friction and other inefficiencies.
Overall, the First Law of Thermodynamics is a powerful tool for analyzing and optimizing thermal systems, such as steam turbine power plants. By understanding the relationships between heat, work, and energy in these systems, engineers can design more efficient and sustainable energy systems to meet the growing demand for clean and reliable energy.
White paper on Thermal Physics First law of thermodynamics
Here is a white paper on the First Law of Thermodynamics in Thermal Physics:
Introduction
The First Law of Thermodynamics is a fundamental principle of thermal physics that describes the behavior of thermal systems. It is based on the principle of conservation of energy, which states that energy cannot be created or destroyed, but can only be converted from one form to another. In the case of thermal systems, energy can be transferred as heat or work. The First Law of Thermodynamics relates the changes in internal energy of a system to the heat added to the system and the work done by the system.
Statement of the First Law of Thermodynamics
The First Law of Thermodynamics can be expressed mathematically as follows:
ΔU = Q – W
where ΔU is the change in internal energy of a system, Q is the heat added to the system, and W is the work done by the system. The law states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Implications of the First Law of Thermodynamics
The First Law of Thermodynamics has a number of important implications for the behavior of thermal systems. Some of these implications are as follows:
Energy Conservation:
The First Law of Thermodynamics states that the total energy of a closed system is conserved. This means that energy cannot be created or destroyed, but can only be transferred from one form to another.
Work and Heat:
The First Law of Thermodynamics relates the changes in internal energy of a system to the work done by the system and the heat added to the system. This means that work and heat are both forms of energy and can be used to transfer energy between different systems.
Efficiency:
The First Law of Thermodynamics can be used to calculate the efficiency of a thermal system. For example, the efficiency of a heat engine can be calculated as the ratio of the work output to the heat input.
Heat Engines:
The First Law of Thermodynamics is fundamental to the study of heat engines, which convert thermal energy into mechanical energy. Heat engines operate by taking in heat from a high-temperature source, converting some of the heat into mechanical work, and then releasing the remaining heat to a low-temperature sink.
Applications of the First Law of Thermodynamics
The First Law of Thermodynamics is used in a wide range of scientific and engineering applications. Some of the applications of the law are as follows:
Heat Engines:
The First Law of Thermodynamics is used to analyze and optimize the performance of heat engines, such as internal combustion engines and gas turbines. By understanding the relationships between heat, work, and energy in these systems, engineers can design more efficient and sustainable energy systems.
Refrigeration and Air Conditioning:
The First Law of Thermodynamics is used in the design of refrigeration and air conditioning systems, which transfer heat from one location to another. By understanding the principles of heat transfer and energy conservation, engineers can design systems that are more efficient and environmentally friendly.
Materials Science:
The First Law of Thermodynamics is used in materials science to study the behavior of materials at high temperatures, such as the melting and solidification of metals. It is also used in the study of phase transitions, such as the transition from a liquid to a gas.
Conclusion
The First Law of Thermodynamics is a fundamental principle of thermal physics that describes the behavior of thermal systems. It is based on the principle of conservation of energy and relates the changes in internal energy of a system to the heat added to the system and the work done by the system. The law has important implications for the behavior of thermal systems, including the efficiency of heat engines, the design of refrigeration and air conditioning systems, and the behavior of materials at high temperatures. Understanding and applying the First Law of Thermodynamics is essential for the development of efficient and sustainable energy systems, as well as for the study of materials science and other fields of science and engineering.