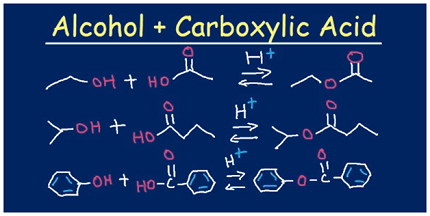
Carboxylic acids can form esters through a reaction called esterification. Esterification involves the reaction of a carboxylic acid with an alcohol in the presence of an acid catalyst. The acid catalyst typically used is concentrated sulfuric acid or hydrochloric acid.
The reaction is typically carried out by combining the carboxylic acid and alcohol in a flask, adding a few drops of the acid catalyst, and heating the mixture under reflux. The reaction proceeds by the acid catalyst protonating the carboxylic acid, which then reacts with the alcohol to form an ester and water.
The general equation for the reaction is:
Carboxylic acid + Alcohol ⇌ Ester + Water
For example, the reaction between acetic acid and ethanol can be represented as:
CH3COOH + C2H5OH ⇌ CH3COOC2H5 + H2O
This reaction is reversible, meaning that the ester can be hydrolyzed back into the carboxylic acid and alcohol under acidic or basic conditions.
Esterification is an important reaction in organic chemistry and is used to synthesize a wide range of esters, which have applications in flavors, fragrances, plastics, and many other industries.
What is Required Carboxylic Acids Formation of Esters
The formation of esters from carboxylic acids requires the presence of an alcohol and an acid catalyst. The acid catalyst is typically concentrated sulfuric acid or hydrochloric acid, which protonates the carboxylic acid and makes it more reactive towards the alcohol.
The reaction also requires heat, which is typically provided through reflux. Reflux is a process in which the reaction mixture is heated to boiling and the vapors are condensed and returned to the reaction vessel. This allows the reaction to continue for an extended period of time and helps drive the equilibrium towards the formation of the ester.
In addition to these requirements, the reaction also requires appropriate stoichiometry between the carboxylic acid and the alcohol. The general equation for the reaction is:
Carboxylic acid + Alcohol ⇌ Ester + Water
The reaction is typically carried out with an excess of the alcohol to shift the equilibrium towards the formation of the ester. The exact amount of alcohol required depends on the specific carboxylic acid being used and the desired yield of the ester product.
Overall, the formation of esters from carboxylic acids is a versatile and important reaction in organic chemistry with many practical applications in industry and everyday life.
When is Required Carboxylic Acids Formation of Esters
The formation of esters from carboxylic acids is required in various situations in organic chemistry, as esters have numerous applications in industry and everyday life. Some examples of when carboxylic acid esterification is required include:
- Flavor and fragrance industry: Many flavors and fragrances are derived from esters, such as the fruity scent of banana or the floral scent of jasmine. Carboxylic acid esterification is used to synthesize these compounds.
- Polymer industry: Esters are used in the production of various polymers, such as polyesters, which are commonly used in clothing, carpet fibers, and other textiles. Carboxylic acid esterification is used to synthesize the ester monomers required for polymerization.
- Pharmaceutical industry: Esters are used as intermediates in the synthesis of many pharmaceuticals, such as aspirin. Carboxylic acid esterification is used to synthesize these intermediates.
- Biochemistry: In biochemistry, esters are involved in many cellular processes, such as the synthesis of lipids and the storage of energy. Carboxylic acid esterification is involved in the synthesis of these esters.
Overall, carboxylic acid esterification is a versatile and important reaction in organic chemistry with many practical applications in various industries and fields of study.
Where is Required Carboxylic Acids Formation of Esters
Carboxylic acid esterification is required in various locations where the production of esters is necessary for specific applications. Some examples of where carboxylic acid esterification is used include:
- Chemical manufacturing facilities: Many chemical manufacturing facilities produce esters for use in various industries, such as the flavor and fragrance industry, polymer industry, and pharmaceutical industry.
- Food and beverage production facilities: Esters are commonly used in the production of flavorings and fragrances for food and beverages, such as artificial strawberry flavoring. Carboxylic acid esterification is used to produce these esters.
- Research and development laboratories: Carboxylic acid esterification is an important reaction in organic chemistry research and is often used in laboratory settings to synthesize new compounds and test their properties.
- Biotechnology and pharmaceutical research facilities: Carboxylic acid esterification is used in the synthesis of many compounds used in biotechnology and pharmaceutical research, such as drugs and drug intermediates.
Overall, carboxylic acid esterification is used in various locations where the production of esters is required for specific applications, such as in the production of flavorings, fragrances, polymers, and pharmaceuticals.
How is Required Carboxylic Acids Formation of Esters
The formation of esters from carboxylic acids involves a reaction known as esterification. The reaction typically involves combining a carboxylic acid with an alcohol in the presence of an acid catalyst, such as concentrated sulfuric acid or hydrochloric acid.
The general equation for the reaction is:
Carboxylic acid + Alcohol ⇌ Ester + Water
The reaction is typically carried out by combining the carboxylic acid and alcohol in a flask, adding a few drops of the acid catalyst, and heating the mixture under reflux. Reflux is a process in which the reaction mixture is heated to boiling and the vapors are condensed and returned to the reaction vessel. This allows the reaction to continue for an extended period of time and helps drive the equilibrium towards the formation of the ester.
During the reaction, the acid catalyst protonates the carboxylic acid, making it more reactive towards the alcohol. The carboxylic acid then reacts with the alcohol to form an ester and water. The reaction is typically carried out with an excess of the alcohol to shift the equilibrium towards the formation of the ester.
The reaction is reversible, meaning that the ester can be hydrolyzed back into the carboxylic acid and alcohol under acidic or basic conditions.
Overall, carboxylic acid esterification is a versatile and important reaction in organic chemistry with many practical applications in various industries and fields of study.
Nomenclature of Carboxylic Acids Formation of Esters
The nomenclature of carboxylic acids and esters follows the rules established by the International Union of Pure and Applied Chemistry (IUPAC).
Carboxylic acids are named using the suffix “-oic acid”. For example, the carboxylic acid with the formula CH3COOH is named acetic acid.
When naming esters, the first part of the name comes from the alcohol and the second part comes from the carboxylic acid. The suffix “-ate” is added to the name of the alcohol, and the prefix derived from the name of the carboxylic acid is added before the “-ate” suffix. For example, if the ester is formed from methanol and acetic acid, it is named methyl acetate.
If the carboxylic acid has a common name, it is used in the name of the ester. For example, the ester formed from ethanol and formic acid is commonly known as ethyl formate, rather than ethyl methanoate.
If the ester contains multiple identical functional groups, the prefix “di-” or “tri-” is added before the name of the functional group to indicate the number of groups present. For example, if the ester contains two ethyl groups, it is named diethyl carbonate.
In summary, the nomenclature of carboxylic acids and esters follows IUPAC rules, with carboxylic acids named using the suffix “-oic acid” and esters named using the name of the alcohol and the carboxylic acid, with the suffix “-ate” added to the alcohol and the carboxylic acid prefix added before the “-ate” suffix.
Case Study on Carboxylic Acids Formation of Esters
One example of the practical application of carboxylic acid esterification is in the production of biodiesel, a renewable and environmentally friendly alternative to conventional diesel fuel. Biodiesel is typically produced by reacting vegetable oil or animal fats with an alcohol, usually methanol, in the presence of a catalyst, such as sodium hydroxide or potassium hydroxide.
The reaction involves a transesterification process in which the triglycerides present in the vegetable oil or animal fats are converted to methyl esters of fatty acids, which are the main component of biodiesel. The reaction can be written as:
Triglyceride + Methanol → Methyl Ester + Glycerol
The reaction proceeds via several steps, with the first step being the hydrolysis of the triglyceride to form fatty acids and glycerol. The fatty acids then react with methanol in the presence of a catalyst to form the corresponding methyl esters and glycerol.
The esterification reaction can also be used to produce flavors and fragrances, which are commonly used in the food and cosmetic industries. For example, ethyl butyrate, which has a fruity odor and is used in the production of artificial fruit flavors, can be produced by reacting butyric acid with ethanol in the presence of an acid catalyst.
Overall, carboxylic acid esterification is a versatile and important reaction with many practical applications in various industries and fields of study. Its ability to form esters, which are widely used in the production of biodiesel, flavors, fragrances, and other compounds, makes it an important reaction for both academic and industrial research.
White paper on Carboxylic Acids Formation of Esters
Introduction
Carboxylic acid esterification is a versatile reaction that is widely used in various industries, including the food, pharmaceutical, and chemical industries. The reaction involves the conversion of a carboxylic acid and an alcohol to an ester and water, and is typically catalyzed by an acid catalyst such as sulfuric acid or hydrochloric acid. In this white paper, we will explore the various aspects of carboxylic acid esterification, including the reaction mechanism, factors that influence the reaction, and its practical applications.
Reaction Mechanism
The esterification of a carboxylic acid and an alcohol involves a reversible reaction that is catalyzed by an acid catalyst. The reaction mechanism can be divided into two steps: the protonation of the carboxylic acid, and the nucleophilic attack of the alcohol on the protonated carboxylic acid. The overall reaction can be written as:
Carboxylic acid + Alcohol ⇌ Ester + Water
In the first step of the reaction, the acid catalyst protonates the carboxylic acid, forming a more reactive species that is susceptible to nucleophilic attack by the alcohol. The protonated carboxylic acid then undergoes nucleophilic attack by the alcohol, forming a tetrahedral intermediate. The tetrahedral intermediate then collapses, leading to the formation of the ester and water.
Factors Influencing the Reaction
Several factors can influence the rate and yield of carboxylic acid esterification, including the type of acid catalyst, the reaction temperature, and the molar ratio of the reactants. Sulfuric acid and hydrochloric acid are commonly used acid catalysts in the reaction, with sulfuric acid being more effective at low temperatures and hydrochloric acid being more effective at high temperatures. The reaction temperature is also an important factor, with higher temperatures generally leading to faster reaction rates. The molar ratio of the reactants is also important, with an excess of alcohol typically used to drive the reaction towards the formation of the ester.
Practical Applications
Carboxylic acid esterification has numerous practical applications in various industries. One of the most important applications is in the production of biodiesel, a renewable and environmentally friendly alternative to conventional diesel fuel. Biodiesel is typically produced by reacting vegetable oil or animal fats with an alcohol, usually methanol, in the presence of a catalyst such as sodium hydroxide or potassium hydroxide. The reaction involves a transesterification process in which the triglycerides present in the vegetable oil or animal fats are converted to methyl esters of fatty acids, which are the main component of biodiesel.
Carboxylic acid esterification is also used in the production of flavors and fragrances, which are commonly used in the food and cosmetic industries. For example, ethyl butyrate, which has a fruity odor and is used in the production of artificial fruit flavors, can be produced by reacting butyric acid with ethanol in the presence of an acid catalyst.
Conclusion
In conclusion, carboxylic acid esterification is a versatile and important reaction that has many practical applications in various industries. Its ability to form esters, which are widely used in the production of biodiesel, flavors, fragrances, and other compounds, makes it an important reaction for both academic and industrial research. The reaction mechanism and factors that influence the reaction are well understood, making it possible to optimize the reaction conditions for specific applications.
