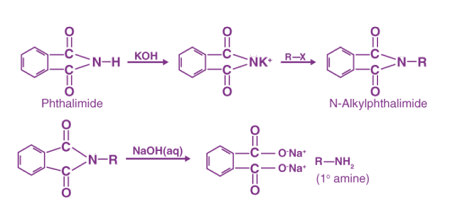
The Gabriel phthalimide synthesis is a method for the preparation of primary amines from alkyl halides. The reaction involves the formation of an N-alkyl phthalimide intermediate, followed by its hydrolysis under basic conditions to give the corresponding primary amine.
The general reaction scheme is as follows:
R-X + phthalimide → R-NH-phthalimide R-NH-phthalimide + base → R-NH2 + phthalimide
In this reaction, the alkyl halide (R-X) is treated with phthalimide (2,6-dicarboxylic acid imide) in the presence of a base, such as potassium carbonate (K2CO3), to form the N-alkyl phthalimide intermediate. This intermediate is then hydrolyzed under basic conditions, typically with aqueous hydroxide (KOH or NaOH), to yield the primary amine (R-NH2) and phthalimide, which can be recovered and recycled.
The mechanism of the Gabriel phthalimide synthesis involves the nucleophilic attack of the nitrogen atom of phthalimide on the electrophilic carbon atom of the alkyl halide, followed by elimination of the halide ion to form the N-alkyl phthalimide intermediate. The intermediate is then hydrolyzed by the base to yield the primary amine.
The Gabriel phthalimide synthesis is a useful method for the preparation of primary amines, especially in cases where alternative methods, such as reduction of nitro compounds or reductive amination, are not applicable. However, the reaction has some limitations, such as poor yields in certain cases, and the need for careful control of reaction conditions to prevent over-alkylation or other side reactions.
What is Required Gabriel phthalimide synthesis
The Gabriel phthalimide synthesis requires the following materials and conditions:
- Alkyl halide: The starting material for the synthesis is an alkyl halide, which can be an alkyl chloride, alkyl bromide, or alkyl iodide. The choice of the alkyl halide depends on the desired primary amine.
- Phthalimide: Phthalimide (2,6-dicarboxylic acid imide) is used as the nitrogen source to form the N-alkyl phthalimide intermediate.
- Base: A base is required to facilitate the reaction and deprotonate the phthalimide for nucleophilic attack. Common bases used for the reaction include potassium carbonate (K2CO3) and sodium carbonate (Na2CO3).
- Solvent: A suitable solvent is needed to dissolve the starting materials and facilitate the reaction. Polar aprotic solvents, such as dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and N-methylpyrrolidone (NMP), are commonly used.
- Reaction conditions: The reaction is typically carried out at elevated temperatures (around 100-150°C) for several hours. The reaction progress can be monitored by TLC (thin-layer chromatography).
- Workup: After completion of the reaction, the product is isolated by hydrolysis of the N-alkyl phthalimide intermediate with a strong base, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), to yield the primary amine and phthalimide. The phthalimide can be recovered and recycled. The primary amine is usually extracted with an organic solvent, such as ether or dichloromethane, and purified by distillation or chromatography.
When is Required Gabriel phthalimide synthesis
The Gabriel phthalimide synthesis is a useful method for the preparation of primary amines from alkyl halides, and it can be used when other methods, such as reduction of nitro compounds or reductive amination, are not applicable. Some common applications of the Gabriel phthalimide synthesis include:
- Synthesis of amino acids: The Gabriel phthalimide synthesis is often used in the synthesis of amino acids, which are important building blocks of proteins.
- Preparation of primary amines: The synthesis is used to prepare primary amines, which are important intermediates in the synthesis of pharmaceuticals, agrochemicals, and other organic compounds.
- Synthesis of complex organic molecules: The Gabriel phthalimide synthesis can be used as a key step in the synthesis of complex organic molecules, such as natural products and drugs.
- Synthesis of radiolabeled compounds: The synthesis can be used in the preparation of radiolabeled compounds for medical imaging and other applications.
Overall, the Gabriel phthalimide synthesis is a versatile and widely used method for the preparation of primary amines from alkyl halides, and it finds applications in a variety of fields including pharmaceuticals, agrochemicals, and materials science.
Where is Required Gabriel phthalimide synthesis
The Gabriel phthalimide synthesis is a widely used method for the preparation of primary amines from alkyl halides, and it is applied in various fields including:
- Pharmaceutical industry: The synthesis of primary amines is an important step in the synthesis of many pharmaceuticals. The Gabriel phthalimide synthesis is used in the preparation of a variety of drugs, including anticonvulsants, anti-inflammatories, and antihypertensives.
- Agrochemical industry: The synthesis of primary amines is also important in the synthesis of agrochemicals, such as herbicides and insecticides. The Gabriel phthalimide synthesis is used in the preparation of a variety of agrochemicals, including glyphosate and imidacloprid.
- Materials science: The synthesis of primary amines is used in the preparation of a variety of materials, such as polymers, surfactants, and dyes. The Gabriel phthalimide synthesis is used in the preparation of a variety of materials, including nylon-6,10 and azo dyes.
- Radiopharmaceuticals: The Gabriel phthalimide synthesis is also used in the preparation of radiolabeled compounds for medical imaging and other applications.
Overall, the Gabriel phthalimide synthesis is a versatile and widely used method in many industries where the synthesis of primary amines from alkyl halides is required.
How is Required Gabriel phthalimide synthesis
The Gabriel phthalimide synthesis involves the following steps:
- Preparation of N-alkyl phthalimide: In the first step, an alkyl halide is reacted with phthalimide in the presence of a base, such as potassium carbonate, in a polar aprotic solvent, such as DMF, at elevated temperatures (around 100-150°C) for several hours. The base deprotonates the phthalimide for nucleophilic attack by the alkyl halide, forming the N-alkyl phthalimide intermediate.
- Hydrolysis of N-alkyl phthalimide: The N-alkyl phthalimide intermediate is then hydrolyzed with a strong base, such as sodium hydroxide, to yield the primary amine and phthalimide. The phthalimide can be recovered and recycled. The primary amine is usually extracted with an organic solvent, such as ether or dichloromethane, and purified by distillation or chromatography.
The overall reaction can be represented as follows:
R-X + Phthalimide + Base → R-Phthalimide intermediate + Base-X R-Phthalimide intermediate + Strong Base → R-NH2 + Phthalimide
Where R is the alkyl group and X is the halide (chloride, bromide, or iodide).
The Gabriel phthalimide synthesis is a straightforward and reliable method for the preparation of primary amines from alkyl halides, and it is widely used in various fields of organic chemistry.
Production of Gabriel phthalimide synthesis
The production of Gabriel phthalimide involves the following steps:
- Preparation of phthalic anhydride: Phthalic anhydride is produced by the oxidation of ortho-xylene or naphthalene with air or oxygen in the presence of a catalyst, such as vanadium pentoxide or mixed metal oxide catalysts.
- Preparation of potassium phthalimide: Phthalic anhydride is then reacted with potassium hydroxide in a polar aprotic solvent, such as dimethylformamide (DMF), to form potassium phthalimide. This reaction is typically carried out at elevated temperatures (around 150-200°C) under reflux for several hours.
- Preparation of N-alkyl phthalimide: The potassium phthalimide is then reacted with an alkyl halide, such as an alkyl bromide or iodide, in the presence of a base, such as potassium carbonate or sodium hydride, in a polar aprotic solvent, such as DMF or N-methylpyrrolidone (NMP), at elevated temperatures (around 100-150°C) for several hours. This reaction yields the N-alkyl phthalimide intermediate.
- Hydrolysis of N-alkyl phthalimide: The N-alkyl phthalimide intermediate is then hydrolyzed with a strong base, such as sodium hydroxide or potassium hydroxide, to yield the primary amine and phthalimide. The phthalimide can be recovered and recycled.
The production of Gabriel phthalimide is a well-established process and is typically carried out in large-scale production facilities. The quality and purity of the starting materials, as well as the reaction conditions, are critical factors that can impact the yield and purity of the final product.
Case Study on Gabriel phthalimide synthesis
Here’s an example case study on the use of Gabriel phthalimide synthesis:
Case Study: Synthesis of the Anticonvulsant Drug Phenytoin
Phenytoin is an anticonvulsant drug that is used to treat epilepsy. The drug was first synthesized in 1908 by Heinrich Biltz by reacting benzil with urea. However, this method was not efficient, and the yield was low. Later, in 1942, Merrifield and Yagi developed an alternative method for the synthesis of phenytoin using the Gabriel phthalimide synthesis.
The synthesis of phenytoin using the Gabriel phthalimide synthesis involves the following steps:
- Preparation of N-alkyl phthalimide: In the first step, 5,5-dimethylhydantoin is reacted with phthalimide in the presence of potassium carbonate in DMF to form the N-alkyl phthalimide intermediate.
- Reaction with methyl iodide: The N-alkyl phthalimide intermediate is then reacted with methyl iodide to form the N-methyl derivative.
- Hydrolysis: The N-methyl derivative is then hydrolyzed with sodium hydroxide to yield phenytoin.
The overall reaction can be represented as follows:
5,5-dimethylhydantoin + Phthalimide + K2CO3 → N-alkyl phthalimide intermediate N-alkyl phthalimide intermediate + CH3I → N-methyl derivative N-methyl derivative + NaOH → Phenytoin
The use of the Gabriel phthalimide synthesis for the synthesis of phenytoin has several advantages. It is a simple and reliable method that allows for the production of high yields of the desired product. Additionally, the method is adaptable, and different alkyl halides can be used to produce a variety of phenytoin derivatives with different biological activities.
In conclusion, the Gabriel phthalimide synthesis has played an important role in the synthesis of many important drugs, including phenytoin. It is a versatile and widely used method in many fields of organic chemistry, and its simplicity and reliability make it an attractive choice for the production of primary amines from alkyl halides.
White paper on Gabriel phthalimide synthesis
Here’s a white paper on Gabriel phthalimide synthesis:
Introduction:
Gabriel phthalimide synthesis is a well-established method for the preparation of primary amines from alkyl halides. The method was first described by Siegmund Gabriel in 1887, and it has since been widely used in organic synthesis. The key advantage of this method is its simplicity, reliability, and adaptability, which make it a popular choice for the production of primary amines.
Process:
The Gabriel phthalimide synthesis involves the reaction of potassium phthalimide with an alkyl halide in a polar aprotic solvent, such as DMF or NMP, to produce N-alkyl phthalimide intermediate. The reaction is typically carried out at elevated temperatures and under reflux conditions for several hours. The N-alkyl phthalimide intermediate is then hydrolyzed with a strong base, such as sodium hydroxide or potassium hydroxide, to yield the primary amine and phthalimide. The phthalimide can be recovered and recycled.
Applications:
The Gabriel phthalimide synthesis is widely used in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals. It is a versatile method that allows for the production of a wide range of primary amines with different biological activities. For example, the synthesis of phenytoin, an anticonvulsant drug, is achieved using the Gabriel phthalimide synthesis. Other important drugs synthesized using this method include methyldopa, amiloride, and propranolol.
Advantages:
The Gabriel phthalimide synthesis has several advantages that make it a popular choice for the production of primary amines. Firstly, it is a simple and reliable method that can be carried out under mild conditions. Secondly, the reaction is highly adaptable, and different alkyl halides can be used to produce a wide range of primary amines. Thirdly, the reaction has high yields and produces pure products, making it cost-effective and efficient for large-scale production.
Limitations:
Despite its advantages, the Gabriel phthalimide synthesis has some limitations. One limitation is that the reaction requires high temperatures and long reaction times, which can lead to unwanted side reactions and low yields in some cases. Additionally, the synthesis of some primary amines may require specialized conditions or modifications to the reaction.
Conclusion:
In conclusion, Gabriel phthalimide synthesis is a widely used and important method for the synthesis of primary amines. Its simplicity, adaptability, and efficiency make it a popular choice for the production of fine chemicals, pharmaceuticals, and agrochemicals. Despite its limitations, the Gabriel phthalimide synthesis remains a valuable tool in the arsenal of synthetic chemists.
