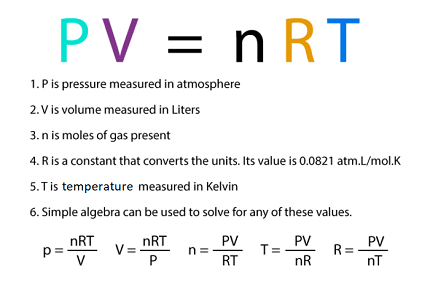
Gas laws describe the behavior of gases under different conditions, such as temperature, pressure, and volume. There are several gas laws, including Boyle’s law, Charles’s law, Gay-Lussac’s law, and the combined gas law, that relate these variables to one another.
Boyle’s law states that at constant temperature, the volume of a gas is inversely proportional to its pressure. In other words, if the pressure on a gas is increased, its volume will decrease, and vice versa.
Charles’s law states that at constant pressure, the volume of a gas is directly proportional to its temperature. This means that as the temperature of a gas increases, its volume also increases, and vice versa.
Gay-Lussac’s law states that at constant volume, the pressure of a gas is directly proportional to its temperature. This means that as the temperature of a gas increases, its pressure also increases, and vice versa.
The combined gas law combines all three of these laws into one equation that relates pressure, volume, and temperature:
(P₁V₁)/T₁ = (P₂V₂)/T₂
where P is pressure, V is volume, and T is temperature. The subscripts 1 and 2 represent the initial and final states of the gas.
The ideal gas equation, also known as the equation of state, describes the behavior of an ideal gas:
PV = nRT
where P is pressure, V is volume, n is the number of moles of gas, R is the universal gas constant, and T is temperature. The ideal gas equation is a combination of Boyle’s law, Charles’s law, and Avogadro’s law, and applies to ideal gases at low pressures and high temperatures.
What is Required Gas laws and ideal gas equation
The required information for applying gas laws and the ideal gas equation includes the values of pressure, volume, temperature, and number of moles of gas.
Gas laws such as Boyle’s law, Charles’s law, and Gay-Lussac’s law require at least two of these variables to be known in order to calculate the third. For example, if the pressure and volume of a gas are known, Charles’s law can be used to determine the gas’s temperature.
The combined gas law incorporates all three variables (pressure, volume, and temperature) and can be used to calculate the final state of a gas if the initial state is known.
The ideal gas equation requires all four variables (pressure, volume, temperature, and number of moles of gas) to be known. The equation can be rearranged to solve for any of the variables, or used to calculate changes in one or more of the variables when the others are changed.
It is important to note that the gas laws and ideal gas equation apply to ideal gases under specific conditions. Deviations from ideal gas behavior can occur at high pressures or low temperatures, or with gases that have strong intermolecular forces or non-negligible volumes. In these cases, the ideal gas equation may need to be modified to account for these deviations.
Who is Required Gas laws and ideal gas equation
Gas laws and the ideal gas equation are fundamental concepts in the study of thermodynamics and the behavior of gases. They are important in many fields of science and engineering, including chemistry, physics, materials science, and mechanical engineering.
The gas laws were first formulated by scientists such as Robert Boyle, Jacques Charles, and Joseph Louis Gay-Lussac in the 17th and 18th centuries. These laws were later combined into the ideal gas law by Émile Clapeyron in the mid-19th century.
The ideal gas equation was further developed by other scientists, including Rudolf Clausius and James Clerk Maxwell, who contributed to the kinetic theory of gases and the understanding of the behavior of gases at the molecular level.
Today, the gas laws and ideal gas equation are used in many applications, such as in the design of engines, the study of atmospheric phenomena, and the production and use of industrial gases. They are also important in understanding and predicting the behavior of gases in chemical reactions and processes.
When is Required Gas laws and ideal gas equation
Gas laws and the ideal gas equation are used in a wide range of situations where gases are involved. Some examples of when gas laws and the ideal gas equation are used include:
- In the design and operation of engines, such as in internal combustion engines and gas turbines. The ideal gas equation is used to calculate the efficiency and performance of these engines.
- In the study of atmospheric phenomena, such as the behavior of air masses and weather patterns. The gas laws are used to understand the changes in pressure, temperature, and volume that occur in the atmosphere.
- In the production and use of industrial gases, such as in the manufacture of steel and other metals. The gas laws are used to control the pressure, volume, and temperature of gases in industrial processes.
- In the study of chemical reactions and processes involving gases. The ideal gas equation is used to calculate the amount of gas produced or consumed in a reaction, as well as to predict the behavior of gases in different conditions.
- In the study of materials science, where gases are often used in the production of materials such as ceramics and semiconductors. The gas laws are used to control the pressure and temperature of gases during these processes.
Overall, the gas laws and ideal gas equation are important tools for understanding the behavior of gases in a wide range of applications, from the operation of engines to the study of chemical reactions and materials science.
Where is Required Gas laws and ideal gas equation
Gas laws and the ideal gas equation are used in a variety of fields and applications, including:
- In chemical laboratories, where gas laws and the ideal gas equation are used to measure and analyze gases. For example, gas chromatography and gas analysis techniques rely on the gas laws to determine the properties of gases.
- In engineering, where gas laws and the ideal gas equation are used to design and operate systems that involve gases, such as engines, turbines, and chemical reactors.
- In meteorology and atmospheric science, where gas laws and the ideal gas equation are used to study the behavior of the atmosphere and predict weather patterns.
- In the production and use of industrial gases, such as in the manufacture of steel and other materials.
- In medical settings, where gas laws and the ideal gas equation are used to monitor and control the gases used in anesthesia and respiratory therapy.
- In environmental science, where gas laws and the ideal gas equation are used to study the behavior of gases in the atmosphere and the impact of human activities on the environment.
Overall, gas laws and the ideal gas equation are used in many different fields and settings where gases are involved, including chemistry, physics, engineering, materials science, and environmental science.
How is Required Gas laws and ideal gas equation
Gas laws and the ideal gas equation are used in a variety of ways depending on the specific application. Here are some examples of how they are used:
- Gas laws can be used to predict the behavior of gases in different conditions. For example, Boyle’s law can be used to predict how the volume of a gas will change as pressure is applied, while Charles’s law can be used to predict how the temperature of a gas will change as its volume changes.
- The ideal gas equation can be used to calculate the pressure, volume, temperature, or number of moles of a gas under different conditions. For example, if the volume and temperature of a gas are known, the ideal gas equation can be used to calculate the pressure of the gas.
- Gas laws and the ideal gas equation are used in the design and operation of various systems that involve gases. For example, in the operation of a gas turbine engine, gas laws and the ideal gas equation are used to optimize the performance of the engine and ensure safe operation.
- In laboratory settings, gas laws and the ideal gas equation are used to measure and analyze gases. For example, gas chromatography relies on the principles of gas laws to separate and analyze different components of a gas mixture.
- Gas laws and the ideal gas equation are used in the study of chemical reactions and processes involving gases. For example, the ideal gas equation can be used to calculate the amount of gas produced or consumed in a chemical reaction, while the gas laws can be used to predict the behavior of gases in different reaction conditions.
Overall, gas laws and the ideal gas equation are used in a variety of ways to understand and predict the behavior of gases, and to design and operate systems that involve gases.
Case Study on Gas laws and ideal gas equation
Let’s consider a case study on the application of gas laws and the ideal gas equation in the design and operation of a gas turbine engine.
A gas turbine engine is a type of internal combustion engine that converts the energy from fuel combustion into mechanical energy to drive a generator or a propulsion system. The operation of a gas turbine engine relies on the principles of thermodynamics and gas laws to optimize its performance and efficiency.
The gas turbine engine consists of three main components: the compressor, the combustion chamber, and the turbine. The compressor compresses the air entering the engine to increase its pressure and temperature. The compressed air is then mixed with fuel in the combustion chamber and ignited to produce a high-temperature, high-pressure gas. The hot gas expands through the turbine, which converts the energy of the gas into mechanical energy to drive a generator or propulsion system.
The operation of a gas turbine engine relies on the ideal gas equation, which relates the pressure, volume, temperature, and number of moles of a gas. The ideal gas equation can be used to calculate the efficiency and performance of the engine under different conditions.
For example, the efficiency of the gas turbine engine can be calculated using the ideal gas equation and the first law of thermodynamics. The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. The efficiency of the gas turbine engine is defined as the ratio of the work output to the heat input.
The efficiency of the gas turbine engine can be improved by increasing the compression ratio of the compressor, which increases the pressure and temperature of the air entering the engine. This results in a higher temperature and pressure in the combustion chamber, which leads to a more efficient combustion process and higher turbine output.
Gas laws, such as Boyle’s law and Charles’s law, are also used in the design and operation of the gas turbine engine. Boyle’s law states that the volume of a gas is inversely proportional to its pressure at constant temperature, while Charles’s law states that the volume of a gas is directly proportional to its temperature at constant pressure. These laws can be used to optimize the performance of the engine by controlling the pressure, volume, and temperature of the gases at different stages of the operation.
In summary, the gas laws and ideal gas equation are essential tools in the design and operation of gas turbine engines, and in many other applications involving gases. They are used to optimize the performance and efficiency of the engine, and to predict the behavior of gases under different conditions.
White paper on Gas laws and ideal gas equation
Introduction:
Gas laws and the ideal gas equation are fundamental concepts in thermodynamics and are used to describe the behavior of gases under different conditions. The study of gas laws and the ideal gas equation is essential in a variety of scientific and engineering fields, including chemistry, physics, and mechanical engineering. In this white paper, we will explore the gas laws and the ideal gas equation in detail and their applications in various fields.
Gas Laws:
Gas laws are a set of fundamental laws that describe the behavior of gases under different conditions. There are three main gas laws: Boyle’s law, Charles’s law, and Gay-Lussac’s law.
Boyle’s Law: Boyle’s law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. This law is expressed mathematically as PV = k, where P is the pressure, V is the volume, and k is a constant.
Charles’s Law: Charles’s law states that at a constant pressure, the volume of a gas is directly proportional to its temperature. This law is expressed mathematically as V/T = k, where V is the volume, T is the temperature, and k is a constant.
Gay-Lussac’s Law: Gay-Lussac’s law states that at a constant volume, the pressure of a gas is directly proportional to its temperature. This law is expressed mathematically as P/T = k, where P is the pressure, T is the temperature, and k is a constant.
Ideal Gas Equation:
The ideal gas equation is a fundamental equation that relates the pressure, volume, temperature, and number of moles of a gas. The ideal gas equation is expressed mathematically as PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
The ideal gas equation is based on the assumptions that the gas molecules have no volume and do not interact with each other. In reality, gases do have volume, and gas molecules interact with each other. However, the ideal gas equation is a good approximation for many gases under normal conditions.
Applications:
Gas laws and the ideal gas equation are used in various scientific and engineering fields, including chemistry, physics, and mechanical engineering. Here are some of the applications of gas laws and the ideal gas equation:
- Gas turbines: Gas turbines are used in power generation and aircraft propulsion systems. Gas laws and the ideal gas equation are used to optimize the performance of gas turbines by controlling the pressure, volume, and temperature of the gases at different stages of the operation.
- Gas chromatography: Gas chromatography is a technique used to separate and analyze different components of a gas mixture. Gas laws are used to predict the behavior of the gases in the chromatography column, while the ideal gas equation is used to calculate the amount of gas produced or consumed in the reaction.
- Chemical reactions: Gas laws and the ideal gas equation are used in the study of chemical reactions involving gases. The ideal gas equation can be used to calculate the amount of gas produced or consumed in a chemical reaction, while gas laws can be used to predict the behavior of gases in different reaction conditions.
- Atmospheric pressure: Gas laws are used to explain the behavior of atmospheric pressure, which is the pressure exerted by the Earth’s atmosphere on objects on the surface of the Earth.
Conclusion:
In conclusion, gas laws and the ideal gas equation are fundamental concepts in thermodynamics and are used to describe the behavior of gases under different conditions. They have applications in various scientific and engineering fields, including chemistry, physics, and mechanical engineering. The study of gas laws and the ideal gas equation is essential for understanding and predicting the behavior of gases and designing systems that involve gases.
