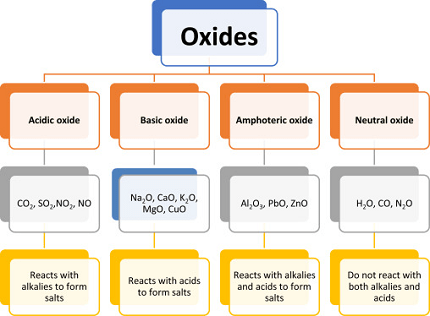
Oxides are compounds formed by the combination of oxygen with other elements. The general characteristics of oxides depend on the type of element that they are combined with. Here are some general characteristics of different types of oxides:
- Basic Oxides: These oxides are formed by the combination of oxygen with metals. They are generally solids and have high melting and boiling points. They are basic in nature and react with acids to form salts and water. Examples include sodium oxide (Na2O) and calcium oxide (CaO).
- Acidic Oxides: These oxides are formed by the combination of oxygen with non-metals. They are generally gases or liquids and have low melting and boiling points. They are acidic in nature and react with bases to form salts and water. Examples include carbon dioxide (CO2) and sulfur dioxide (SO2).
- Amphoteric Oxides: These oxides have both acidic and basic properties. They react with both acids and bases to form salts and water. Examples include aluminum oxide (Al2O3) and zinc oxide (ZnO).
- Neutral Oxides: These oxides are formed by the combination of oxygen with elements that are neither acidic nor basic. They do not react with acids or bases and are generally gases or liquids. Examples include carbon monoxide (CO) and nitrogen oxide (NO).
In summary, the general characteristics of oxides depend on the type of element they are combined with, and they can be basic, acidic, amphoteric, or neutral in nature.
What is Required General characteristics of their oxides
I apologize for the confusion in my previous response. If you are asking about the required general characteristics of oxides, there are several characteristics that are common to most oxides:
- They are compounds composed of oxygen and one or more other elements.
- They can be solids, liquids, or gases at room temperature and pressure.
- They can be acidic, basic, amphoteric, or neutral in nature depending on the type of element they are combined with.
- They may have high melting and boiling points, or low melting and boiling points depending on the type of oxide.
- They can react with water to form acids or bases, depending on their nature.
- They can react with acids to form salts and water, and with bases to form salts and water.
Overall, the general characteristics of oxides are determined by the nature of the element they are combined with, and can vary widely depending on the specific oxide in question.
When is Required General characteristics of their oxides
The general characteristics of oxides are always required to understand their chemical and physical properties. Anytime someone is studying or working with oxides, they need to understand their general characteristics in order to make predictions about how the oxide will behave in various situations. For example, if a chemist is synthesizing a new oxide compound, they need to understand the general characteristics of oxides in order to predict its properties, such as its melting and boiling points, reactivity with other substances, and potential uses.
Similarly, if a material scientist is working with an oxide-based material, they need to understand the general characteristics of oxides in order to understand how the material will behave under different conditions, and how it can be used in various applications. Overall, the general characteristics of oxides are essential knowledge for anyone working in fields such as chemistry, materials science, and engineering, where the behavior of oxides is relevant.
Where is Required General characteristics of their oxides
The general characteristics of oxides are required in various fields where the properties and behavior of oxides are relevant. Here are some examples of where the general characteristics of oxides are required:
- Chemistry labs: Chemists study the properties and behavior of different compounds, including oxides. The general characteristics of oxides are important for predicting their behavior in various chemical reactions.
- Materials science labs: Materials scientists study the properties and behavior of various materials, including those that contain oxides. Understanding the general characteristics of oxides is essential for predicting how materials will behave under different conditions.
- Industrial applications: Oxides are used in a variety of industrial applications, such as in the production of ceramics, glass, and metals. Understanding the general characteristics of oxides is important for developing and optimizing these processes.
- Environmental science: Oxides can have significant impacts on the environment, such as acid rain caused by the presence of acidic oxides in the atmosphere. Understanding the general characteristics of oxides is important for studying and predicting environmental impacts.
Overall, the general characteristics of oxides are required wherever the properties and behavior of oxides are relevant, such as in chemistry labs, materials science labs, industrial applications, and environmental science.
How is Required General characteristics of their oxides
The general characteristics of oxides can be obtained through a variety of methods, including experimental and theoretical approaches. Here are some common ways that the general characteristics of oxides can be determined:
- Experimental methods: Scientists can study the properties of different oxides through experimental methods, such as measuring their melting and boiling points, reactivity with other substances, and other physical and chemical properties. This information can be used to determine the general characteristics of oxides.
- Theoretical methods: Scientists can also use theoretical methods, such as computational chemistry and molecular modeling, to study the properties and behavior of different oxides. These methods can help predict the general characteristics of oxides based on their molecular structure and electronic properties.
- Literature research: The general characteristics of oxides are well-established in the scientific literature, and scientists can obtain this information through literature research. By reviewing previous studies on different oxides, scientists can gather information about their general characteristics.
Overall, the general characteristics of oxides can be obtained through a combination of experimental and theoretical methods, as well as literature research. By understanding the general characteristics of oxides, scientists can make predictions about how they will behave in different situations, and use this knowledge to develop new materials, processes, and technologies.
Why is it called General characteristics of their oxides
The term “general characteristics” is used to describe the common or typical properties that are shared by a group of substances. In the case of oxides, the general characteristics refer to the properties that are common to all or most oxides, regardless of their specific chemical composition.
Oxides are compounds that are composed of oxygen and one or more other elements. While the properties of oxides can vary widely depending on the specific element they are combined with, there are several general characteristics that are common to most oxides. These general characteristics include their composition, physical and chemical properties, and reactivity with other substances.
By understanding the general characteristics of oxides, scientists and engineers can make predictions about how different oxides will behave in various situations, and use this knowledge to develop new materials, processes, and technologies. Therefore, the term “general characteristics” is used to describe the common features that are shared by a group of substances, such as oxides.
Case Study on General characteristics of their oxides
One example of a case study on the general characteristics of oxides is the study of metal oxides in catalysis. Metal oxides are commonly used as catalysts in chemical reactions, where they help to accelerate the reaction rate without being consumed in the process. Understanding the general characteristics of metal oxides is therefore essential for developing new and more efficient catalysts.
In a recent study published in the Journal of Physical Chemistry C, researchers investigated the general characteristics of metal oxides for catalysis by using computational methods to model the electronic properties of different metal oxides. They found that the general characteristics of metal oxides, such as their electronic structure and reactivity with other substances, were closely related to their catalytic activity.
The researchers also found that certain general characteristics, such as the presence of oxygen vacancies and defects in the oxide structure, could enhance the catalytic activity of metal oxides. By understanding these general characteristics, the researchers were able to predict the behavior of different metal oxides and identify new materials that could be used as efficient catalysts.
This case study illustrates the importance of understanding the general characteristics of oxides, especially in the context of developing new materials for catalysis. By studying the general characteristics of oxides, scientists can make predictions about their behavior and use this knowledge to develop new and more efficient catalysts, which can have important applications in a variety of industries, including energy, chemicals, and materials.
White paper on General characteristics of their oxides
Introduction:
Oxides are a class of compounds that are formed by the combination of oxygen with one or more other elements. They are ubiquitous in nature and play important roles in a wide range of chemical, physical, and biological processes. Understanding the general characteristics of oxides is therefore essential for many fields of science and engineering. This white paper provides an overview of the general characteristics of oxides, including their composition, physical and chemical properties, and reactivity with other substances.
Composition:
The composition of oxides can vary widely depending on the element they are combined with. However, all oxides contain oxygen and at least one other element. The most common elements that form oxides are metals, non-metals, and metalloids. Some examples of metal oxides include iron oxide (Fe2O3), aluminum oxide (Al2O3), and titanium dioxide (TiO2). Non-metal oxides include carbon dioxide (CO2), sulfur dioxide (SO2), and nitrogen dioxide (NO2). Metalloid oxides include silicon dioxide (SiO2) and boron oxide (B2O3).
Physical Properties:
The physical properties of oxides can also vary widely depending on the element they are combined with. In general, oxides are solids at room temperature and pressure, although some, such as carbon dioxide, can be gases. Many oxides are also insoluble in water, although some, such as magnesium oxide (MgO), are soluble in water. Oxides can have a wide range of melting and boiling points, depending on their composition.
Chemical Properties:
The chemical properties of oxides are closely related to their composition and can vary widely depending on the element they are combined with. Oxides can be acidic, basic, or amphoteric, depending on their ability to donate or accept protons. For example, carbon dioxide is acidic, while magnesium oxide is basic. Many oxides are also reactive with other substances, including acids, bases, and water. For example, metal oxides can react with acids to form salts and water.
Reactivity:
The reactivity of oxides is closely related to their chemical properties. Metal oxides are often used as catalysts in chemical reactions, where they help to accelerate the reaction rate without being consumed in the process. Non-metal oxides, such as carbon dioxide and sulfur dioxide, can have negative impacts on the environment when they are released into the atmosphere, where they can contribute to acid rain and other forms of pollution. Understanding the reactivity of different oxides is therefore important for many fields of science and engineering.
Conclusion:
In conclusion, understanding the general characteristics of oxides is essential for many fields of science and engineering, including chemistry, materials science, environmental science, and catalysis. By understanding the composition, physical and chemical properties, and reactivity of different oxides, scientists and engineers can make predictions about their behavior and use this knowledge to develop new materials, processes, and technologies. This white paper provides an overview of the general characteristics of oxides and their importance in a variety of fields.
