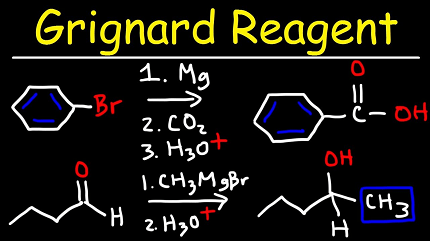
Grignard reactions are a class of organic reactions that involve the addition of an organomagnesium halide (a Grignard reagent) to a carbonyl group in an organic compound. The reaction was discovered by French chemist François Auguste Victor Grignard in 1900, and he was awarded the Nobel Prize in Chemistry in 1912 for this work.
Grignard reagents are typically prepared by reacting an alkyl or aryl halide with magnesium metal in the presence of an ether solvent. The resulting Grignard reagent is a powerful nucleophile that can react with a wide variety of electrophiles, including carbonyl compounds, epoxides, and halogens.
The reaction mechanism involves the formation of a complex between the Grignard reagent and the carbonyl compound, followed by nucleophilic addition to the carbonyl carbon. This forms an alkoxide intermediate, which can then be protonated to give the final product.
Grignard reactions have a wide range of synthetic applications, including the synthesis of alcohols, alkanes, alkenes, and alkynes. They are also used in the synthesis of natural products, pharmaceuticals, and agrochemicals. However, Grignard reactions can be sensitive to moisture and air, and require careful handling and purification techniques.
What is Required Alkyl Halides Grignard reactions
Alkyl halides, particularly primary alkyl halides, are the most commonly used starting materials in Grignard reactions. These alkyl halides can be prepared by reacting an alkyl alcohol with a hydrogen halide, such as hydrochloric acid or hydrobromic acid. Alternatively, they can be prepared by reacting an alkene with hydrogen halide in the presence of a peroxide initiator (known as the HBr peroxide reaction).
Secondary and tertiary alkyl halides can also be used in Grignard reactions, but they are generally less reactive than primary alkyl halides due to steric hindrance. In addition, vinyl halides and aryl halides can also be used in Grignard reactions.
It is important to note that the choice of alkyl halide can affect the reaction yield and selectivity. For example, some alkyl halides may react too slowly or give undesired side products. Therefore, the selection of the appropriate alkyl halide is critical for the success of the Grignard reaction.
When is Required Alkyl Halides Grignard reactions
Alkyl halides are required in Grignard reactions as they serve as the starting material for the formation of the Grignard reagent. The Grignard reagent is formed by the reaction of the alkyl halide with magnesium metal in the presence of an ether solvent. The resulting Grignard reagent is a powerful nucleophile that can react with a wide variety of electrophiles, including carbonyl compounds, epoxides, and halogens.
The choice of alkyl halide can affect the reaction yield and selectivity. For example, primary alkyl halides are the most commonly used starting materials in Grignard reactions due to their higher reactivity compared to secondary or tertiary alkyl halides, which are hindered by steric effects. Additionally, vinyl halides and aryl halides can also be used in Grignard reactions.
Grignard reactions have a wide range of synthetic applications, including the synthesis of alcohols, alkanes, alkenes, and alkynes. They are also used in the synthesis of natural products, pharmaceuticals, and agrochemicals. However, Grignard reactions can be sensitive to moisture and air, and require careful handling and purification techniques.
Where is Required Alkyl Halides Grignard reactions
Grignard reactions using alkyl halides as starting materials can be carried out in a variety of laboratory settings, including academic and industrial research laboratories. The reactions are typically performed under an inert atmosphere, such as nitrogen or argon, to prevent the Grignard reagent from reacting with moisture or oxygen in the air.
The reaction is usually carried out in a flask equipped with a reflux condenser and a magnetic stirrer. The flask is first purged with inert gas and dried using a drying agent, such as calcium chloride, before the solvent and the alkyl halide are added. The magnesium turnings are then added, followed by gradual addition of the ether solvent. The reaction is initiated by adding a small amount of iodine or another suitable initiator.
After the reaction is complete, the mixture is quenched with a protic solvent, such as water or ethanol, to destroy any remaining Grignard reagent. The product is then extracted with an organic solvent and purified using various techniques, such as column chromatography or recrystallization.
Grignard reactions using alkyl halides as starting materials can be used to synthesize a wide range of organic compounds, including alcohols, alkanes, alkenes, and alkynes, which have many industrial and academic applications.
How is Required Alkyl Halides Grignard reactions
The synthesis of Grignard reagents using alkyl halides involves the reaction of the alkyl halide with magnesium metal in the presence of an ether solvent. The reaction proceeds as follows:
- Preparation of the reaction flask: The reaction flask is dried and purged with an inert gas, such as nitrogen or argon, to prevent moisture and oxygen from reacting with the Grignard reagent. A reflux condenser and a magnetic stirrer are attached to the flask.
- Preparation of the alkyl halide: The alkyl halide is prepared by reacting an alkyl alcohol with a hydrogen halide, such as hydrochloric acid or hydrobromic acid. Alternatively, it can be prepared by reacting an alkene with hydrogen halide in the presence of a peroxide initiator (known as the HBr peroxide reaction).
- Formation of the Grignard reagent: The alkyl halide is added to the reaction flask containing magnesium turnings and ether solvent. The reaction is initiated by adding a small amount of iodine or another suitable initiator. The reaction proceeds through a radical mechanism, with the alkyl halide undergoing a single electron transfer to the magnesium metal, generating an alkyl radical and a magnesium halide intermediate. The alkyl radical then reacts with another molecule of the alkyl halide to form the Grignard reagent.
- Reaction with an electrophile: The Grignard reagent can react with a wide range of electrophiles, including carbonyl compounds, epoxides, and halogens. The reaction proceeds via nucleophilic addition of the Grignard reagent to the electrophilic center, followed by protonation to give the final product.
- Quenching and workup: After the reaction is complete, the mixture is quenched with a protic solvent, such as water or ethanol, to destroy any remaining Grignard reagent. The product is then extracted with an organic solvent and purified using various techniques, such as column chromatography or recrystallization.
Overall, the synthesis of Grignard reagents using alkyl halides is a powerful and versatile method for the formation of carbon-carbon bonds in organic synthesis. The reaction requires careful handling and purification techniques due to its sensitivity to moisture and air.
Production of Alkyl Halides Grignard reactions
Alkyl halides can be synthesized using a variety of methods, including the addition of a halogen to an alkene, the substitution of a leaving group in an alkyl compound, or the addition of a hydrogen halide to an alkene. Once the alkyl halide is synthesized, it can be used as a starting material for Grignard reactions.
One common method for synthesizing alkyl halides is the free-radical halogenation of alkanes. This reaction involves the use of a halogen, such as chlorine or bromine, in the presence of heat or light to replace a hydrogen atom in an alkane with a halogen atom. For example, the reaction of methane with chlorine gas yields chloromethane:
CH4 + Cl2 → CH3Cl + HCl
In the laboratory, alkyl halides can also be synthesized through the reaction of an alcohol with a hydrogen halide, such as HCl or HBr. The reaction is typically carried out in the presence of a catalyst, such as zinc chloride, to promote the formation of the alkyl halide. For example, the reaction of ethanol with hydrochloric acid yields ethyl chloride:
CH3CH2OH + HCl → CH3CH2Cl + H2O
Alkyl halides can also be synthesized through the substitution of a leaving group in an alkyl compound, such as an alkyl chloride or alkyl sulfate. This reaction typically involves the use of a strong nucleophile, such as hydroxide or cyanide, to replace the leaving group. For example, the reaction of ethyl chloride with sodium hydroxide yields ethanol:
CH3CH2Cl + NaOH → CH3CH2OH + NaCl
Once the alkyl halide is synthesized, it can be used as a starting material for the synthesis of Grignard reagents, which can then be used for a variety of organic reactions.
Case Study on Alkyl Halides Grignard reactions
One application of Grignard reactions involving alkyl halides is the synthesis of alcohols. For example, the synthesis of tert-butyl alcohol from tert-butyl bromide is a classic example of a Grignard reaction.
The reaction proceeds as follows:
- Formation of the Grignard reagent: tert-butyl bromide is reacted with magnesium turnings and an ether solvent, such as diethyl ether, to form tert-butyl magnesium bromide, the Grignard reagent.
- Reaction with formaldehyde: The Grignard reagent is then reacted with formaldehyde (HCHO) to yield tert-butyl alcohol. The reaction proceeds via nucleophilic addition of the Grignard reagent to the carbonyl group of formaldehyde, followed by protonation to give the final product.
The reaction can be written as:
CH3C(CH3)2Br + Mg → CH3C(CH3)2MgBr
CH3C(CH3)2MgBr + HCHO → CH3C(CH3)2OH + MgBr(OCH2CH3)
The synthesis of tert-butyl alcohol via the Grignard reaction is an important demonstration of the power of this method for the formation of carbon-carbon bonds and the synthesis of complex organic molecules. The reaction is sensitive to moisture and air, so it requires careful handling and purification techniques to obtain high yields of the desired product. However, with proper attention to detail and a well-equipped laboratory, the Grignard reaction can be a valuable tool for synthetic chemists.
White paper on Alkyl Halides Grignard reactions
Introduction:
The Grignard reaction is a powerful synthetic tool used to form carbon-carbon bonds, and alkyl halides are commonly used as starting materials for Grignard reactions. In this white paper, we will discuss the mechanism of Grignard reactions involving alkyl halides, their applications in organic synthesis, and some considerations for carrying out these reactions in the laboratory.
Mechanism of Alkyl Halides Grignard Reactions:
The reaction of an alkyl halide with magnesium in the presence of an ether solvent, such as diethyl ether, leads to the formation of a Grignard reagent. The Grignard reagent is an organometallic compound that contains a carbon-magnesium bond, and it is highly reactive due to the polarized carbon-magnesium bond.
The reaction proceeds via a series of steps, as follows:
- Formation of the Grignard reagent: The alkyl halide reacts with magnesium in the presence of an ether solvent to form the Grignard reagent.
- Nucleophilic addition: The Grignard reagent acts as a nucleophile and attacks an electrophilic carbon atom, such as the carbonyl carbon of an aldehyde or ketone.
- Formation of an alkoxide intermediate: The nucleophilic attack leads to the formation of an alkoxide intermediate, which is stabilized by the ether solvent.
- Protonation: The alkoxide intermediate is protonated by a proton source, such as water or an acid, to yield the final product.
Applications of Alkyl Halides Grignard Reactions:
The Grignard reaction is a versatile tool for organic synthesis, and it can be used to synthesize a wide variety of organic compounds, including alcohols, ketones, aldehydes, carboxylic acids, and esters. The reaction is particularly useful for the synthesis of complex organic molecules that contain multiple functional groups, as it allows for the formation of multiple carbon-carbon bonds in a single step.
One common application of the Grignard reaction involving alkyl halides is the synthesis of alcohols. For example, the reaction of ethyl bromide with magnesium and an ether solvent followed by the addition of water yields ethanol:
CH3CH2Br + Mg → CH3CH2MgBr CH3CH2MgBr + H2O → CH3CH2OH + Mg(OH)Br
Considerations for Carrying Out Alkyl Halides Grignard Reactions:
The Grignard reaction involving alkyl halides is highly exothermic, and it can be dangerous if not carried out properly. The reaction is also sensitive to moisture and air, which can quench the reaction and reduce yields. Therefore, careful handling and purification techniques are necessary to obtain high yields of the desired product.
In the laboratory, Grignard reactions involving alkyl halides are typically carried out under an inert atmosphere, such as nitrogen or argon, to prevent exposure to moisture and air. The reaction vessel and all glassware should be thoroughly dried and evacuated to remove any traces of moisture or air. The reaction should be monitored carefully, and additional magnesium or alkyl halide may be added as needed to maintain the reaction. Once the reaction is complete, the product is typically purified by a combination of techniques, such as distillation or chromatography.
Conclusion:
In conclusion, the Grignard reaction involving alkyl halides is an important synthetic tool in organic chemistry. It allows for the formation of carbon-carbon bonds and the synthesis of a wide range of organic compounds, including alcohols, ketones, aldehydes, carboxylic acids, and esters. However, the reaction requires careful handling and purification techniques due to its sensitivity to moisture and air. With proper attention to detail and a well-equipped laboratory, the Grignard reaction can be a powerful tool for organic synthesis.
