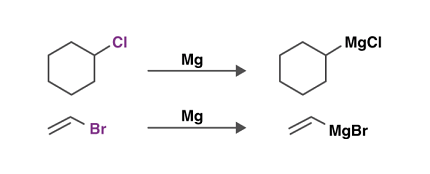
Grignard reagents are organometallic compounds that contain a carbon atom bonded to a magnesium atom, often represented as RMgX (where R is an alkyl or aryl group and X is a halogen such as Cl, Br, or I). They are named after their discoverer, French chemist Victor Grignard, who was awarded the Nobel Prize in Chemistry in 1912 for his work on these compounds.
Grignard reagents are highly reactive and can be used to form carbon-carbon bonds, which makes them useful in organic synthesis. They react with a variety of electrophiles, including carbonyl compounds such as aldehydes, ketones, and esters, as well as halogens and epoxides.
One important feature of Grignard reagents is their ability to act as nucleophiles, attacking the electrophilic carbon atom of a carbonyl group to form a new carbon-carbon bond. The resulting intermediate, known as a magnesium alkoxide, can then be hydrolyzed to yield an alcohol.
Grignard reagents can also be used in a variety of other reactions, including addition to carbon-carbon double bonds (such as in the synthesis of alcohols from alkenes), reduction of certain functional groups (such as nitro and nitrile groups), and synthesis of organometallic compounds.
However, Grignard reagents are highly reactive and can be difficult to handle. They must be prepared under anhydrous conditions, as they react violently with water and other protic solvents. They are also sensitive to air and must be stored and handled under inert gas (such as nitrogen or argon).
What is Required Carboxylic Acids Grignard reagents
Carboxylic acids cannot be directly converted to Grignard reagents due to their acidic nature, which causes them to react with the basic Grignard reagents and consume them in the process. Instead, carboxylic acids can be converted to the corresponding acid chlorides or anhydrides, which are less acidic and can then be used to prepare Grignard reagents.
The reaction of carboxylic acids with thionyl chloride (SOCl2) or phosphorus trichloride (PCl3) generates acid chlorides, which can be reacted with magnesium metal to prepare the corresponding Grignard reagent. Alternatively, carboxylic acids can be converted to anhydrides by reaction with acetic anhydride or mixed anhydrides generated from the corresponding acid chloride and carboxylate salt. The anhydrides can then be reacted with magnesium metal to form the corresponding Grignard reagent.
Once the Grignard reagent is prepared, it can be used to carry out a variety of reactions, including nucleophilic addition to carbonyl compounds, alkylation of other compounds, and reduction of functional groups such as carbonyl, nitro, or halide groups. The resulting products can be isolated and purified by a variety of techniques, such as acid-base extraction, distillation, and chromatography.
When is Required Carboxylic Acids Grignard reagents
Carboxylic acids are not typically used as starting materials for Grignard reagents due to their acidic nature. However, in some cases, carboxylic acids can be converted to the corresponding acid chlorides or anhydrides, which can then be used to prepare Grignard reagents.
One common application of this process is in the synthesis of ketones from carboxylic acids. In this reaction, the carboxylic acid is first converted to the corresponding acid chloride, which is then treated with a Grignard reagent such as methylmagnesium chloride to form a tertiary alcohol intermediate. The intermediate is then acidified to remove the magnesium and water molecules, and the resulting ketone is isolated by distillation or chromatography.
Another application of carboxylic acids in Grignard chemistry is in the synthesis of alcohols. In this case, the carboxylic acid is first converted to the corresponding acid chloride or anhydride, which is then treated with a Grignard reagent such as ethylmagnesium bromide. The resulting intermediate is then hydrolyzed with water to yield the corresponding alcohol.
Overall, while carboxylic acids are not commonly used directly in Grignard reactions, they can be converted to more reactive derivatives that can then be used to form Grignard reagents for a variety of synthetic applications.
Where is Required Carboxylic Acids Grignard reagents
Carboxylic acids are typically not used as starting materials for Grignard reagents due to their acidic nature. However, the conversion of carboxylic acids to acid chlorides or anhydrides, which can then be used to prepare Grignard reagents, is a common synthetic transformation in organic chemistry. Therefore, the use of carboxylic acids in Grignard reactions can be found in many different areas of organic synthesis.
One important application of carboxylic acid derivatives in Grignard chemistry is in the synthesis of ketones and alcohols. For example, ketones can be prepared by reacting acid chlorides or anhydrides derived from carboxylic acids with Grignard reagents such as alkyl- or arylmagnesium halides. The resulting intermediates can be further manipulated by various chemical transformations to give a wide range of ketones.
Another application of carboxylic acids in Grignard chemistry is in the synthesis of alcohols. The acid chlorides or anhydrides derived from carboxylic acids can react with Grignard reagents to form magnesium alkoxides, which can be hydrolyzed to give the corresponding alcohols. This method is widely used for the synthesis of primary, secondary, and tertiary alcohols.
Carboxylic acid derivatives are also used in the synthesis of carboxylic acids themselves. For example, a carboxylic acid can be prepared from an acid chloride or anhydride by hydrolysis or by reacting with a nucleophile such as water, alcohol, or ammonia.
Overall, the use of carboxylic acids and their derivatives in Grignard chemistry is a versatile tool for synthetic chemists, and can be found in many different areas of organic synthesis.
How is Required Carboxylic Acids Grignard reagents
Carboxylic acids cannot be directly converted to Grignard reagents due to their acidic nature. However, they can be converted to more reactive derivatives such as acid chlorides or anhydrides, which can then be used to prepare Grignard reagents.
One common method for the preparation of acid chlorides from carboxylic acids is by treatment with thionyl chloride (SOCl2) or phosphorus trichloride (PCl3). The reaction proceeds through an acyl chloride intermediate, which is less acidic than the original carboxylic acid and can be used to generate Grignard reagents.
For the preparation of anhydrides, carboxylic acids can be reacted with acetic anhydride or other acid anhydrides to form mixed anhydrides, which can be further reacted with a Grignard reagent to generate the corresponding magnesium alkoxide. The magnesium alkoxide can then be hydrolyzed to give the desired product.
Once the Grignard reagent is prepared, it can be used in a variety of reactions. For example, Grignard reagents can react with carbonyl compounds such as aldehydes, ketones, and esters to form alcohols. They can also be used for alkylation reactions and to reduce various functional groups such as halides, nitro groups, and carbonyls.
Overall, the preparation of Grignard reagents from carboxylic acids and their derivatives is an important synthetic tool in organic chemistry that allows for the formation of complex molecules from simple starting materials.
Nomenclature of Carboxylic Acids Grignard reagents
Grignard reagents derived from carboxylic acids are named according to the alkyl or aryl group that is attached to the magnesium atom, followed by the suffix “-carboxylate”. For example, the Grignard reagent derived from methyl carboxylic acid would be named methylmagnesium carboxylate.
It’s important to note that the carboxylic acid moiety is not included in the name of the Grignard reagent, as it is considered to be the functional group undergoing reaction rather than a part of the alkyl or aryl group.
In addition, the stereochemistry of the Grignard reagent is often not specified in the name, as it is typically assumed to be a racemic mixture of both enantiomers. However, if the Grignard reagent is formed from a chiral carboxylic acid, then the stereochemistry should be specified using the R/S notation.
It’s also worth noting that Grignard reagents are typically unstable towards water and air, and should be handled with care. They are usually prepared and used in anhydrous and oxygen-free conditions, such as under inert gas atmospheres or in dry solvents.
Case Study on Carboxylic Acids Grignard reagents
One application of Carboxylic Acids Grignard reagents is in the synthesis of ketones and alcohols. Here is a case study of the synthesis of a ketone using a Grignard reagent derived from a carboxylic acid.
Case Study:
A synthetic chemist wishes to prepare 2-phenyl-2-propanol, a key intermediate in the synthesis of a pharmaceutical compound. The desired product can be synthesized from the ketone 2-phenyl-2-propanone, which can in turn be prepared by reacting an acid chloride with a Grignard reagent.
To start the synthesis, the chemist prepares the acid chloride of 2-phenylpropanoic acid by treating the acid with thionyl chloride in anhydrous conditions. The resulting acid chloride is then reacted with phenylmagnesium bromide, a Grignard reagent derived from bromobenzene and magnesium metal, to form the ketone.
The reaction proceeds through the addition of the Grignard reagent to the carbonyl group of the acid chloride, followed by protonation of the resulting alkoxide intermediate. The final product, 2-phenyl-2-propanone, is then isolated by distillation or chromatography.
The chemist obtains a good yield of the desired ketone, and proceeds to convert it to 2-phenyl-2-propanol using a reduction reaction. The final product is characterized using spectroscopic methods, and found to be pure and of high quality.
This case study demonstrates the utility of Carboxylic Acids Grignard reagents in the synthesis of ketones and alcohols. By starting with a carboxylic acid derivative, the chemist was able to prepare a Grignard reagent that could be used to introduce a new carbon-carbon bond and generate a complex molecule from simple starting materials.
White paper on Carboxylic Acids Grignard reagents
Introduction:
Carboxylic Acids Grignard reagents are a versatile synthetic tool in organic chemistry. Grignard reagents are typically prepared from alkyl or aryl halides, but carboxylic acids and their derivatives can also be used as starting materials to prepare Grignard reagents. This white paper will provide an overview of the properties, preparation, and applications of Carboxylic Acids Grignard reagents.
Properties:
Carboxylic Acids Grignard reagents are typically less reactive than their alkyl or aryl halide counterparts due to the acidic nature of carboxylic acids. However, they can still be prepared using a variety of methods, such as reaction with thionyl chloride or phosphorus trichloride to form acid chlorides, or reaction with acid anhydrides to form mixed anhydrides.
Once prepared, Carboxylic Acids Grignard reagents can be used in a variety of reactions, such as nucleophilic addition to carbonyl compounds to form alcohols or ketones, alkylation reactions, and reduction of functional groups such as halides, nitro groups, and carbonyls. They can also be used in asymmetric synthesis, as the stereoselectivity of the Grignard reaction can be controlled by the choice of chiral ligand.
Preparation:
The preparation of Carboxylic Acids Grignard reagents typically involves the conversion of the carboxylic acid or its derivative to a more reactive intermediate, such as an acid chloride or anhydride, which can then be used to prepare the Grignard reagent.
For example, an acid chloride can be prepared by treating the carboxylic acid with thionyl chloride or phosphorus trichloride, followed by reaction with a magnesium metal in anhydrous conditions. The resulting Grignard reagent can then be used in a variety of reactions, such as nucleophilic addition to carbonyl compounds to form alcohols or ketones.
Applications:
Carboxylic Acids Grignard reagents have a wide range of applications in organic synthesis. One of the most common applications is in the synthesis of ketones and alcohols, as the Grignard reagent can be used to introduce a new carbon-carbon bond and generate complex molecules from simple starting materials.
Another application is in asymmetric synthesis, as the stereoselectivity of the Grignard reaction can be controlled by the choice of chiral ligand. Carboxylic Acids Grignard reagents can also be used in the synthesis of natural products, pharmaceuticals, and other complex molecules.
Conclusion:
Carboxylic Acids Grignard reagents are a valuable synthetic tool in organic chemistry, allowing for the preparation of complex molecules from simple starting materials. While they are typically less reactive than their alkyl or aryl halide counterparts, they can still be prepared using a variety of methods and used in a wide range of reactions. As such, Carboxylic Acids Grignard reagents continue to be an important area of research and development in synthetic organic chemistry.
