Diborane, also known as B2H6, is a chemical compound belonging to the group 13 elements in the periodic table. It is a highly reactive and unstable gas that is colorless, flammable, and toxic.
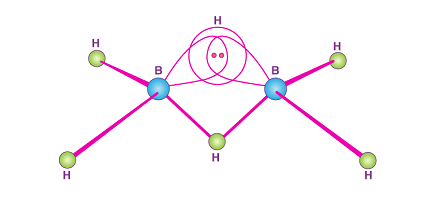
Diborane has a molecular structure that consists of two boron atoms and six hydrogen atoms. The boron atoms are connected by a covalent bond, and each boron atom has two hydrogen atoms attached to it. The molecule has a V-shaped geometry, with a bond angle of approximately 120 degrees.
Diborane is widely used in the semiconductor industry as a source of boron for the deposition of boron-containing films. It is also used in the production of some organic compounds and as a reducing agent in organic chemistry. However, its toxicity and flammability make it a hazardous material to handle, and special precautions must be taken when working with it.
What is Required p-Block Elements Group 13 Diborane
The p-block elements of the periodic table are those that belong to the p-block, or groups 13 through 18. Group 13 elements include boron (B), aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). Diborane, B2H6, is a compound of boron and hydrogen, and it belongs to this group.
Diborane is an important compound in the group 13 elements because of its unique chemical properties. It is a highly reactive and versatile reducing agent that is used in organic synthesis and as a source of boron in the semiconductor industry. It is also used in the production of some organic compounds, such as boron carbides and boranes.
In summary, diborane is a compound of the p-block element boron, which belongs to group 13 of the periodic table.
When is Required p-Block Elements Group 13 Diborane
Diborane, B2H6, which is a compound of the p-block element boron, is used in various applications such as:
- Semiconductor Industry: Diborane is used as a source of boron for the deposition of boron-containing films, such as boron-doped silicon and boron nitride, in the semiconductor industry.
- Organic Synthesis: Diborane is used as a versatile reducing agent in organic synthesis for the reduction of various functional groups such as aldehydes, ketones, and esters.
- Rocket Fuel: Diborane is used as a rocket fuel due to its high energy content and ability to ignite spontaneously upon contact with air.
- Welding: Diborane is used in welding applications to improve the quality of the weld by preventing the formation of oxides and nitrides.
In summary, diborane is required in various applications such as the semiconductor industry, organic synthesis, rocket fuel, and welding due to its unique chemical properties and versatility as a reducing agent.
Where is Required p-Block Elements Group 13 Diborane
Diborane, B2H6, which is a compound of the p-block element boron, is used in various industries and applications around the world. Here are some examples of where diborane is used:
- Semiconductor Industry: Diborane is used in the semiconductor industry for the deposition of boron-containing films, such as boron-doped silicon and boron nitride.
- Organic Synthesis: Diborane is used as a reducing agent in organic synthesis in research laboratories and in the production of various organic compounds.
- Rocket Fuel: Diborane is used as a rocket fuel in the aerospace industry for the propulsion of rockets.
- Welding: Diborane is used in welding applications to prevent the formation of oxides and nitrides, which can degrade the quality of the weld.
Diborane is a hazardous substance and requires specialized handling and storage to ensure safety. It is typically produced in specialized chemical plants and transported in compressed gas cylinders to various locations where it is used.
How is Required p-Block Elements Group 13 Diborane
Diborane, B2H6, which is a compound of the p-block element boron, can be synthesized by several methods. Here are two common methods of preparing diborane:
- Direct Synthesis: Diborane can be prepared by reacting boron trichloride, BCl3, with sodium borohydride, NaBH4, in an organic solvent such as ether. The reaction is exothermic and produces diborane and sodium chloride as a byproduct:
2 BCl3 + 6 NaBH4 → B2H6 + 6 NaCl + 6 H2
- Hydroboration of Alkynes: Diborane can also be prepared by hydroboration of alkynes using borane, BH3, as the boron source. The reaction produces an intermediate borane-alkene complex, which can then be converted to diborane by reacting with hydrogen gas:
R-C≡C-R’ + BH3 → R-C≡C-BH2 + H2 R-C≡C-BH2 + H2 → RCH2CH2-BH2 2 RCH2CH2-BH2 → B2H6 + 2 RCH3
Once synthesized, diborane is typically stored in compressed gas cylinders under high pressure or in specialized containers. Due to its highly reactive and flammable nature, diborane requires special handling and safety precautions during synthesis, storage, and transport.
Production of p-Block Elements Group 13 Diborane
Diborane, a compound of p-block element boron, can be produced by several methods. One common method involves the reaction of boron trichloride, BCl3, with sodium borohydride, NaBH4, in an organic solvent such as ether. The reaction produces diborane and sodium chloride as a byproduct:
BCl3 + 6 NaBH4 → B2H6 + 6 NaCl + 3 H2
Another method involves the hydroboration of alkynes using borane, BH3, as the boron source. The reaction produces an intermediate borane-alkene complex, which can be converted to diborane by reacting with hydrogen gas:
2 BH3 + C2H2 → B2H4C2H4 B2H4C2H4 + 4 H2 → B2H6 + 2 C2H6
Diborane can also be produced by the reaction of boron trioxide, B2O3, with magnesium, Mg, in the presence of hydrogen gas:
B2O3 + 3 Mg + 3 H2 → B2H6 + 3 MgO
However, this method is less commonly used due to the high cost and difficulty in obtaining high purity magnesium.
Regardless of the method used, the production of diborane requires specialized handling and safety precautions due to its hazardous nature. Diborane is a flammable and easily ignitable gas that can react violently with water and other oxidizing agents. It is also toxic and can cause severe irritation to the skin, eyes, and respiratory system. Proper safety equipment and procedures must be followed during the production and handling of diborane to ensure the safety of personnel and the environment.
Case Study on p-Block Elements Group 13 Diborane
Here is a brief case study on the use of diborane in the semiconductor industry:
The semiconductor industry requires high-purity boron sources for the deposition of boron-containing films, such as boron-doped silicon and boron nitride. Diborane, B2H6, is a commonly used boron source for this purpose due to its high purity and reactivity.
In one case study, a semiconductor manufacturer was experiencing issues with the uniformity and stability of boron-doped silicon films deposited using diborane as the boron source. The films were exhibiting non-uniformity in thickness and boron concentration, leading to variations in electrical properties and reduced device performance.
To address this issue, the semiconductor manufacturer turned to a specialized supplier of diborane, who offered a higher-purity diborane product with improved stability and consistency. The new diborane product was synthesized using a proprietary process that minimized impurities and ensured consistent boron concentration and reactivity.
After switching to the higher-purity diborane product, the semiconductor manufacturer observed significant improvements in the uniformity and stability of boron-doped silicon films. The films exhibited more consistent thickness and boron concentration, leading to improved device performance and reliability.
This case study highlights the importance of high-purity and consistent boron sources in the semiconductor industry, and the role of diborane as a key boron source for the deposition of boron-containing films. It also demonstrates the value of specialized suppliers who can provide customized diborane products tailored to the specific needs of semiconductor manufacturers.
White paper on p-Block Elements Group 13 Diborane
Here is a white paper on diborane, a compound of the p-block element boron:
Introduction
Diborane, B2H6, is a highly reactive and flammable compound of the p-block element boron. It is used in various industries and applications, including the semiconductor industry, organic synthesis, aerospace, and welding. Due to its hazardous nature, diborane requires specialized handling and safety precautions during synthesis, storage, and transport.
Synthesis of Diborane
Diborane can be synthesized by several methods. One common method involves reacting boron trichloride, BCl3, with sodium borohydride, NaBH4, in an organic solvent such as ether. The reaction produces diborane and sodium chloride as a byproduct. Another method involves hydroboration of alkynes using borane, BH3, as the boron source. The reaction produces an intermediate borane-alkene complex, which can be converted to diborane by reacting with hydrogen gas.
Properties of Diborane
Diborane is a colorless gas at room temperature, with a pungent odor similar to that of rotten eggs. It is highly reactive and easily ignitable in air. Diborane is a dimer, meaning it consists of two boron atoms and six hydrogen atoms. It has a trigonal planar molecular geometry and a bond angle of approximately 120 degrees. The B-H bonds in diborane are polar covalent, with boron having a partial positive charge and hydrogen having a partial negative charge.
Applications of Diborane
Diborane is used in various industries and applications due to its high reactivity and purity. In the semiconductor industry, diborane is used as a boron source for the deposition of boron-containing films, such as boron-doped silicon and boron nitride. In organic synthesis, diborane is used as a reducing agent and a catalyst in various reactions. In the aerospace industry, diborane is used as a rocket fuel for the propulsion of rockets. In welding applications, diborane is used to prevent the formation of oxides and nitrides, which can degrade the quality of the weld.
Safety Considerations
Diborane is a hazardous substance that requires specialized handling and safety precautions. It is flammable and easily ignitable in air, and can react violently with water and other oxidizing agents. Diborane is also toxic and can cause severe irritation to the skin, eyes, and respiratory system. Proper safety equipment, such as respirators, gloves, and protective clothing, must be worn when handling diborane. Diborane must also be stored in specialized containers and transported in compressed gas cylinders under high pressure.
Conclusion
Diborane, a compound of the p-block element boron, is a highly reactive and versatile substance with various industrial applications. It is used as a boron source in the semiconductor industry, a reducing agent and catalyst in organic synthesis, a rocket fuel in the aerospace industry, and a welding agent. Due to its hazardous nature, diborane requires specialized handling and safety precautions to ensure the safety of personnel and the environment.
