Group 14 of the periodic table is also known as the Carbon Group. It includes the elements carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb). Here are some of the general properties of Group 14 elements:
- Valence electrons: All Group 14 elements have four valence electrons, making them capable of forming covalent bonds with other elements.
- Electronegativity: The electronegativity of the elements in Group 14 decreases as you go down the group. Carbon has the highest electronegativity, while lead has the lowest.
- Reactivity: The reactivity of the Group 14 elements also decreases as you go down the group. Carbon is the most reactive element in the group, and lead is the least reactive.
- Melting and boiling points: The melting and boiling points of the elements in Group 14 generally increase as you go down the group. This is due to the increasing strength of the metallic bonds.
- Allotropes: Carbon is known for its allotropes, including diamond, graphite, and fullerenes. Silicon and germanium also have allotropes.
- Semiconductor properties: Silicon and germanium are semiconductors and are widely used in the electronics industry.
- Toxicity: Lead is a toxic element that can cause serious health problems. It is no longer used in many applications due to its toxicity.
Overall, Group 14 elements have a wide range of properties and applications. Carbon is essential for life and is used in a variety of industries, including construction, electronics, and medicine. Silicon and germanium are critical for the electronics industry, and tin is used in alloys and coatings. Lead, despite its toxicity, has been used in various applications throughout history, including pipes, batteries, and paint.
What is Required p-Block Elements Group 14 Properties
The p-block elements of Group 14 in the periodic table are Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb). Here are some important properties of the Group 14 elements:
- Valence Electrons: All the elements in Group 14 have four valence electrons.
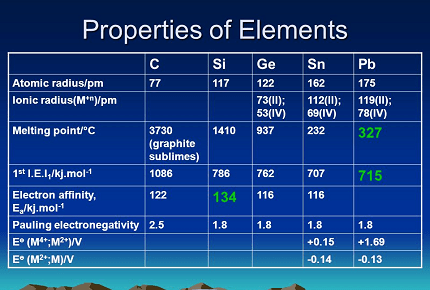
- Atomic Radius: The atomic radius of the elements increases as we go down the group. This is because the number of shells increases as we move down the group.
- Electronegativity: The electronegativity of the elements in Group 14 decreases as we move down the group. This is because the effective nuclear charge decreases and the distance between the nucleus and the valence electrons increases.
- Ionization Energy: The ionization energy of the elements in Group 14 also decreases as we move down the group. This is because the effective nuclear charge decreases, making it easier to remove the outermost electron.
- Melting and Boiling Points: The melting and boiling points of the elements in Group 14 generally increase as we move down the group. This is because of the increasing strength of the metallic bonds.
- Allotropes: Carbon is known for its allotropes, including diamond, graphite, and fullerenes. Silicon and Germanium also have allotropes.
- Semiconductor Properties: Silicon and Germanium are semiconductors and are widely used in the electronics industry.
- Toxicity: Lead is a toxic element that can cause serious health problems. It is no longer used in many applications due to its toxicity.
These properties of Group 14 elements are essential in understanding their behavior and applications in various fields like electronics, construction, medicine, etc.
When is Required p-Block Elements Group 14 Properties
The properties of the p-block elements of Group 14 in the periodic table are required in various fields such as chemistry, physics, materials science, electronics, and engineering.
In chemistry, the properties of Group 14 elements are important in understanding their behavior and reactivity in various chemical reactions. For example, the reactivity of carbon in organic chemistry and the semiconducting properties of silicon and germanium in inorganic chemistry.
In physics, the properties of Group 14 elements are important in understanding their electronic structure, optical properties, and mechanical properties. For example, the electrical conductivity of tin and lead, and the mechanical strength of diamond.
In materials science, the properties of Group 14 elements are important in designing and developing new materials with specific properties. For example, the use of silicon and germanium in the development of semiconductors, and the use of carbon in the development of high-strength materials such as graphene.
In electronics, the semiconducting properties of silicon and germanium are crucial in the development of electronic devices such as transistors, diodes, and solar cells.
In engineering, the properties of Group 14 elements are important in designing and developing new materials and structures with specific properties. For example, the use of lead in the construction of radiation shields and the use of tin in the development of new alloys with specific mechanical properties.
Overall, the properties of Group 14 elements are essential in understanding their behavior and applications in various fields, making them an important area of study in science and engineering.
Where is Required p-Block Elements Group 14 Properties
The p-block elements of Group 14 in the periodic table can be found in the middle of the periodic table, between the s-block and the d-block elements. The p-block elements are located on the right side of the periodic table, and they include groups 13 through 18.
Specifically, Group 14 elements – Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb) – are located in the fourth row of the periodic table. Carbon is located at the top of the group, while lead is located at the bottom.
The properties of Group 14 elements are studied in various fields, including chemistry, physics, materials science, electronics, and engineering. The properties of these elements are important in understanding their behavior and applications in various fields, making them a critical area of study in science and engineering.
How is Required p-Block Elements Group 14 Properties
The properties of Group 14 elements are determined by their electronic configurations and the periodic trends that arise from variations in the effective nuclear charge, atomic radius, and shielding effect across the group. Here are some of the ways that these properties manifest:
- Valence Electrons: All the Group 14 elements have four valence electrons. These electrons are involved in chemical bonding, and the number of valence electrons plays a crucial role in determining the chemical properties of the elements.
- Atomic Radius: The atomic radius of the Group 14 elements increases as we move down the group due to the increase in the number of shells. This results in a larger distance between the nucleus and the outermost electrons, which weakens the attractive force between them.
- Electronegativity: The electronegativity of the Group 14 elements decreases as we move down the group due to the decrease in the effective nuclear charge. This makes it easier for the elements to attract electrons in a chemical bond.
- Ionization Energy: The ionization energy of the Group 14 elements decreases as we move down the group due to the increase in atomic size and the decrease in effective nuclear charge. This means that it is easier to remove an electron from the outer shell of an element as we move down the group.
- Melting and Boiling Points: The melting and boiling points of the Group 14 elements generally increase as we move down the group due to the increasing strength of metallic bonding. Diamond, for example, has a very high melting point due to the strength of its covalent bonds.
- Allotropes: Carbon is known for its allotropes, including diamond, graphite, and fullerenes. Silicon and Germanium also have allotropes. These allotropes have different structures and properties, which can be useful in various applications.
- Semiconductor Properties: Silicon and Germanium are semiconductors, which means that they have intermediate conductivity between that of a conductor and an insulator. This property makes them useful in electronic devices such as transistors.
- Toxicity: Lead is a toxic element that can cause serious health problems. It is no longer used in many applications due to its toxicity.
These properties of Group 14 elements are important in understanding their behavior and applications in various fields, such as chemistry, physics, materials science, electronics, and engineering.
Production of p-Block Elements Group 14 Properties
The production of Group 14 elements involves various processes depending on the specific element and its intended use. Here are some common methods for producing Group 14 elements:
- Carbon: Carbon is the most abundant element in the group and can be found in various forms, including coal, oil, natural gas, and biomass. It can also be produced through the process of pyrolysis, which involves heating organic materials in the absence of oxygen.
- Silicon: Silicon is typically produced through the reduction of silicon dioxide (SiO2) with carbon in an electric furnace. This process is called the “Carbothermic Process.” Another method of producing silicon is through the reduction of silicon tetrachloride (SiCl4) with hydrogen gas (H2) in the presence of a catalyst.
- Germanium: Germanium is typically produced through the purification of flue dust from zinc smelters, which contains a small amount of germanium. It can also be produced through the reduction of germanium dioxide (GeO2) with hydrogen gas (H2) or through the electrolysis of a solution of germanium dioxide in molten sodium hydroxide (NaOH).
- Tin: Tin is typically produced through the smelting of tin ores, such as cassiterite, which is a tin oxide mineral. The smelting process involves heating the ore with carbon in a furnace to produce tin metal and carbon dioxide gas.
- Lead: Lead is typically produced through the smelting of lead ores, such as galena, which is a lead sulfide mineral. The smelting process involves heating the ore with carbon in a furnace to produce lead metal and sulfur dioxide gas.
Overall, the production of Group 14 elements involves various processes, including extraction from ores, reduction of oxides or chlorides, and purification methods. These methods are important in producing high-quality Group 14 elements for various applications in chemistry, physics, materials science, electronics, and engineering.
Case Study on p-Block Elements Group 14 Properties
One example of the application of Group 14 elements is in the field of semiconductor technology, where silicon is a widely used material for electronic devices. Here is a case study that highlights the importance of Group 14 elements in this field:
Case Study: Silicon in Semiconductor Technology
Semiconductor technology is an important field that uses materials with specific properties to create electronic devices, such as transistors, diodes, and integrated circuits. One of the most commonly used materials in this field is silicon, a Group 14 element that has unique properties that make it ideal for use in electronic devices.
Silicon has four valence electrons, which makes it a semiconductor. When a small amount of impurity is added to silicon, it becomes either a p-type or n-type semiconductor. A p-type semiconductor is created when a small amount of a Group 13 element, such as boron, is added to silicon, while an n-type semiconductor is created when a small amount of a Group 15 element, such as phosphorus, is added to silicon.
The use of p-type and n-type semiconductors in electronic devices allows for the creation of p-n junctions, which are important for the operation of devices such as transistors and diodes. Transistors are used in amplifiers and switches, while diodes are used to control the flow of current in a circuit.
In addition to its use in electronic devices, silicon is also used as a substrate material for integrated circuits. The substrate is the base material onto which electronic devices are fabricated. Silicon is an ideal substrate material because it is abundant, easy to produce, and has a high melting point, which makes it resistant to high temperatures.
Overall, the unique properties of silicon, such as its semiconducting properties and its use as a substrate material, make it an essential material in the field of semiconductor technology. The study of Group 14 elements, and their properties, has been critical in the development of this technology, and it continues to play a crucial role in the advancement of electronics and computing.
White paper on p-Block Elements Group 14 Properties
Introduction:
The p-block elements in the periodic table refer to the elements found in groups 13 to 18. Group 14 of the p-block contains carbon, silicon, germanium, tin, and lead. These elements have unique properties that make them essential in many applications, such as in semiconductor technology, metallurgy, and organic chemistry. This white paper will discuss the properties of Group 14 elements, including their electronic configuration, physical properties, chemical reactivity, and applications.
Electronic Configuration:
The Group 14 elements have four valence electrons, which means they have a similar electron configuration. The electronic configuration of these elements is ns2np2, where n is the principal quantum number. The outermost electrons of Group 14 elements are in the p orbital, which makes them good candidates for forming covalent bonds.
Physical Properties:
The physical properties of Group 14 elements vary depending on the specific element. Carbon, for example, is a non-metal and has a very high melting and boiling point. Silicon, on the other hand, is a metalloid and has a lower melting and boiling point than carbon. Germanium is also a metalloid and has a lower melting and boiling point than silicon. Tin is a metal, and its melting and boiling point are lower than those of carbon and silicon. Finally, lead is also a metal, and its melting and boiling point are lower than those of tin.
Chemical Reactivity:
Group 14 elements have different chemical reactivities, but they all have a tendency to form covalent bonds with other atoms. Carbon, for example, forms a variety of organic compounds, such as hydrocarbons, alcohols, and carboxylic acids. Silicon is used extensively in semiconductor technology, and its ability to form covalent bonds with other atoms makes it ideal for creating the p-n junctions necessary for the operation of electronic devices.
Germanium also has semiconductor properties and is used in the production of transistors and diodes. Tin is used in metallurgy, where it is added to other metals to create alloys with desirable properties. Finally, lead has a high density and is used in a variety of applications, such as in batteries, ammunition, and radiation shielding.
Applications:
The Group 14 elements have a wide range of applications, including:
- Carbon: Used in the production of steel, fuel cells, and organic compounds.
- Silicon: Used extensively in semiconductor technology, including in the production of computer chips and solar cells.
- Germanium: Used in the production of transistors, diodes, and infrared optical materials.
- Tin: Used in the production of alloys, such as bronze, pewter, and solder.
- Lead: Used in batteries, ammunition, radiation shielding, and as a component in paint.
Conclusion:
The Group 14 elements are essential in many applications, including in semiconductor technology, metallurgy, and organic chemistry. Their unique properties, including their ability to form covalent bonds and their semiconductor properties, make them ideal for use in electronic devices. The study of Group 14 elements and their properties continues to be critical in the development of new technologies and materials, and they will undoubtedly play an important role in shaping the future of science and engineering.
