Carbon monoxide (CO) is a colorless, odorless, and highly toxic gas. While it is primarily known for its harmful effects on human health, there are also some industrial and medical applications for carbon monoxide. Here are some of the uses of carbon monoxide:
- Production of chemicals: Carbon monoxide is used as a raw material in the production of a variety of chemicals, including methanol, formaldehyde, and acetic acid.
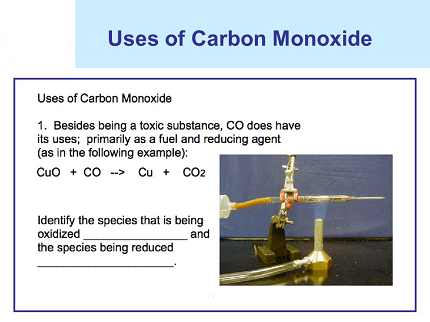
- Reduction of metal ores: Carbon monoxide is used as a reducing agent in the production of iron and steel. It reacts with iron ore to produce iron, and with other metals to produce metal carbonyls, which can be used as catalysts or as intermediates in the production of other chemicals.
- Welding and cutting: Carbon monoxide can be used as a fuel gas for welding and cutting metals. It provides a high-energy flame that can cut through thick metal plates.
- Medical applications: Carbon monoxide can be used in medical treatments, such as hyperbaric oxygen therapy, to help increase oxygen delivery to tissues.
- Chemical synthesis: Carbon monoxide can be used as a reactant in chemical synthesis reactions to produce a wide range of organic compounds.
- Fuel: Carbon monoxide can be used as a fuel in internal combustion engines. However, its toxicity and low energy content make it less desirable than other fuels.
It is important to note that while carbon monoxide has some industrial and medical uses, it is a highly toxic gas that can be lethal in high concentrations. Proper safety precautions must be taken when handling or using carbon monoxide.
What is Required p-Block Elements Group 14 Uses of carbon monoxide
The p-block elements of Group 14 include carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb). Carbon monoxide (CO) is not a Group 14 element, but it is a compound that can be formed from the reaction of Group 14 elements with oxygen. Here are some of the uses of carbon monoxide that are relevant to Group 14 elements:
- Carbon monoxide as a reducing agent: Carbon monoxide can be used as a reducing agent in the extraction of metals such as tin and lead from their ores. The reaction of carbon monoxide with metal oxides produces the corresponding metal and carbon dioxide. For example, tin(IV) oxide (SnO2) can be reduced to tin metal (Sn) by heating with carbon monoxide:
SnO2(s) + CO(g) → Sn(s) + CO2(g)
- Silicon carbide production: Carbon monoxide can be used as a reactant in the production of silicon carbide (SiC), which is used in the manufacturing of cutting tools, abrasives, and refractory materials. The reaction of silicon dioxide (SiO2) with carbon at high temperatures produces silicon carbide and carbon monoxide:
SiO2(s) + 3C(s) → SiC(s) + 2CO(g)
- Synthesis of organic compounds: Carbon monoxide can be used as a reactant in organic synthesis reactions to produce a wide range of organic compounds. For example, the Reppe synthesis is a method for producing organic compounds using carbon monoxide and alkenes (compounds with double bonds):
H2C=CH2 + CO → CH3CHO
Overall, carbon monoxide has several important uses in industrial processes, but it is also a highly toxic gas that can be lethal in high concentrations. Proper safety precautions must be taken when handling or using carbon monoxide.
When is Required p-Block Elements Group 14 Uses of carbon monoxide
The uses of carbon monoxide in relation to the p-block elements of Group 14 have been known for several decades. The industrial production of metals such as tin and lead using carbon monoxide as a reducing agent dates back to the 19th century. Similarly, the synthesis of silicon carbide using carbon monoxide as a reactant has been a known process since the early 20th century.
Carbon monoxide has also been used as a reactant in organic synthesis reactions for many years. For example, the Reppe synthesis, which involves the reaction of carbon monoxide and alkenes to produce organic compounds, was developed in the 1950s.
Overall, the uses of carbon monoxide in relation to Group 14 elements have been well-established for many years, and the processes involved are widely used in industry today. However, it is important to note that carbon monoxide is a highly toxic gas, and proper safety precautions must always be taken when handling or using it.
Where is Required p-Block Elements Group 14 Uses of carbon monoxide
The uses of carbon monoxide in relation to the p-block elements of Group 14 can be found in various industries and applications around the world. Here are some examples of where these uses can be found:
- Metal production: Carbon monoxide is used as a reducing agent in the production of metals such as tin and lead. This process is used in metal refineries and foundries around the world.
- Silicon carbide production: Carbon monoxide is used as a reactant in the production of silicon carbide, which is used in a variety of industrial applications. This process can be found in silicon carbide production facilities around the world.
- Organic synthesis: Carbon monoxide is used as a reactant in organic synthesis reactions to produce a wide range of organic compounds. This process can be found in chemical synthesis labs and in the pharmaceutical industry.
- Welding and cutting: Carbon monoxide can be used as a fuel gas for welding and cutting metals. This process can be found in metal fabrication facilities and in shipyards.
Overall, the uses of carbon monoxide in relation to Group 14 elements can be found in various industrial processes and applications around the world. However, it is important to note that carbon monoxide is a highly toxic gas, and proper safety precautions must always be taken when handling or using it.
Nomenclature of p-Block Elements Group 14 Uses of carbon monoxide
The p-block elements of Group 14 in the periodic table include carbon, silicon, germanium, tin, and lead. These elements are named based on their atomic number, and the prefix “p-” indicates that they belong to the p-block of the periodic table.
Carbon monoxide, which is commonly used in relation to Group 14 elements, is a compound made up of one carbon atom and one oxygen atom. Its chemical formula is CO, and it is named using the prefix “mono-” to indicate one oxygen atom and the suffix “-oxide” to indicate the presence of oxygen. The name of the element carbon is derived from the Latin word “carbo,” which means coal, charcoal, or carbon black.
The other compounds that can be formed by the Group 14 elements and carbon monoxide include metal carbonyls, such as iron pentacarbonyl (Fe(CO)5), which is used in the production of carbonyl iron powder, and nickel tetracarbonyl (Ni(CO)4), which is used as a catalyst in chemical reactions. These compounds are named using the name of the metal followed by the word “carbonyl,” which indicates the presence of carbon monoxide ligands bonded to the metal center.
In summary, the nomenclature of p-block elements of Group 14 is based on their atomic number, while the nomenclature of compounds formed by these elements and carbon monoxide is based on the specific atoms present and their bonding.
How is Required p-Block Elements Group 14 Uses of carbon monoxide
The uses of carbon monoxide in relation to the p-block elements of Group 14 involve various chemical reactions and processes. Here are some examples of how carbon monoxide is used in relation to Group 14 elements:
- Metal production: Carbon monoxide is used as a reducing agent in the production of metals such as tin and lead. The reaction involves the carbon monoxide gas reacting with the metal oxide to produce the corresponding metal and carbon dioxide. For example:
SnO2 + 2CO -> Sn + 2CO2
This reaction takes place at high temperatures and in the presence of a catalyst, such as iron or nickel.
- Silicon carbide production: Carbon monoxide is used as a reactant in the production of silicon carbide. The reaction involves the carbon monoxide gas reacting with silicon dioxide to produce silicon carbide and carbon dioxide. For example:
SiO2 + 3C -> SiC + 2CO
This reaction also takes place at high temperatures and in the presence of a catalyst, such as iron or copper.
- Organic synthesis: Carbon monoxide is used as a reactant in organic synthesis reactions to produce a wide range of organic compounds. For example, in the Reppe synthesis, carbon monoxide reacts with alkenes to produce aldehydes. The reaction can be catalyzed by a variety of transition metals, such as palladium or nickel.
- Welding and cutting: Carbon monoxide can be used as a fuel gas for welding and cutting metals. The gas is mixed with oxygen and ignited to produce a high temperature flame that can melt and cut through metal.
Overall, the uses of carbon monoxide in relation to Group 14 elements involve a variety of chemical reactions and processes that require careful handling and control to ensure safety and optimal results.
Case Study on p-Block Elements Group 14 Uses of carbon monoxide
One example of a case study on the uses of carbon monoxide in relation to the p-block elements of Group 14 is the production of silicon carbide.
Silicon carbide is a compound made up of silicon and carbon, and it has a wide range of industrial applications, including in the production of ceramic materials, abrasive powders, and refractory materials. One of the most common methods for producing silicon carbide is the Acheson process, which uses carbon as a reactant.
However, in recent years, there has been increasing interest in using carbon monoxide as a reactant for silicon carbide production. This is because carbon monoxide has a higher reactivity than carbon, and it can react with silicon dioxide at lower temperatures, which can reduce energy consumption and production costs.
One study published in the Journal of the American Ceramic Society investigated the use of carbon monoxide for silicon carbide production. The researchers used a fluidized bed reactor to carry out the reaction between silicon dioxide and carbon monoxide, and they varied the reaction temperature and gas composition to optimize the process.
The results showed that the use of carbon monoxide resulted in higher silicon carbide yields compared to traditional carbon-based methods. In addition, the use of carbon monoxide allowed for greater control over the reaction parameters, which can lead to more consistent and higher quality silicon carbide products.
Overall, this case study highlights the potential benefits of using carbon monoxide in relation to Group 14 elements, and how this can lead to improvements in industrial processes and applications. However, it is important to note that the use of carbon monoxide requires careful handling and control due to its highly toxic nature.
White paper on p-Block Elements Group 14 Uses of carbon monoxide
Introduction:
Carbon monoxide is a colorless, odorless, and toxic gas that has a wide range of industrial applications, particularly in relation to the p-block elements of Group 14. This white paper aims to provide an overview of the uses of carbon monoxide in relation to Group 14 elements, as well as the benefits and challenges associated with these applications.
Uses of carbon monoxide in relation to Group 14 elements:
- Metal production: Carbon monoxide is used as a reducing agent in the production of metals such as tin and lead. The reaction involves the carbon monoxide gas reacting with the metal oxide to produce the corresponding metal and carbon dioxide.
- Silicon carbide production: Carbon monoxide is used as a reactant in the production of silicon carbide, which has a wide range of industrial applications, including in the production of ceramic materials, abrasive powders, and refractory materials. The reaction involves the carbon monoxide gas reacting with silicon dioxide to produce silicon carbide and carbon dioxide.
- Organic synthesis: Carbon monoxide is used as a reactant in organic synthesis reactions to produce a wide range of organic compounds. For example, in the Reppe synthesis, carbon monoxide reacts with alkenes to produce aldehydes.
- Welding and cutting: Carbon monoxide can be used as a fuel gas for welding and cutting metals. The gas is mixed with oxygen and ignited to produce a high-temperature flame that can melt and cut through metal.
Benefits of using carbon monoxide:
- Higher reactivity: Carbon monoxide has a higher reactivity than carbon, which can lead to higher reaction rates and greater product yields in industrial processes.
- Lower reaction temperatures: Carbon monoxide can react with some metal oxides and silicon dioxide at lower temperatures compared to traditional carbon-based methods, which can reduce energy consumption and production costs.
- Greater control: The use of carbon monoxide allows for greater control over reaction parameters, which can lead to more consistent and higher quality products.
Challenges associated with using carbon monoxide:
- Toxicity: Carbon monoxide is a highly toxic gas that can cause serious health problems, including headaches, nausea, and even death. Proper safety precautions must always be taken when handling or using carbon monoxide.
- Environmental concerns: Carbon monoxide is a greenhouse gas that can contribute to climate change. Its use in industrial processes must be carefully regulated to minimize environmental impact.
Conclusion:
The uses of carbon monoxide in relation to Group 14 elements have a wide range of applications in various industries around the world. The benefits of using carbon monoxide, including higher reactivity, lower reaction temperatures, and greater control over reaction parameters, make it an attractive option for many industrial processes. However, the toxicity and environmental concerns associated with carbon monoxide use must be carefully addressed to ensure safe and sustainable industrial practices.
