Phosphorus is a chemical element with the symbol P and atomic number 15. It has several allotropes, which are different forms of the same element that exist in the same physical state.
The most common allotropes of phosphorus are:
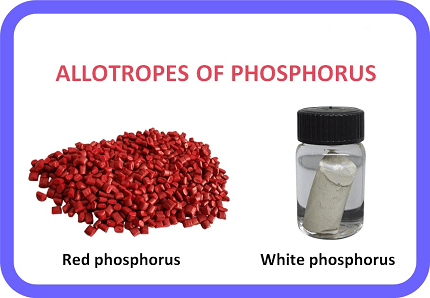
- White phosphorus: It is a waxy, yellowish-white, highly reactive, and toxic substance that ignites spontaneously in air. It has a tetrahedral molecular structure and is the most reactive form of phosphorus.
- Red phosphorus: It is a dark red powder that is less reactive than white phosphorus. It has a polymeric structure and is used in the manufacture of matches, fireworks, and semiconductors.
- Black phosphorus: It is a black, crystalline solid that is less reactive than white phosphorus. It has a layered structure and is a semiconductor.
- Violet phosphorus: It is a violet or purple-colored solid that is metastable and only exists at high pressures. It has a structure similar to black phosphorus but with a different arrangement of atoms.
- Hittorf’s metallic phosphorus: It is a metallic form of phosphorus that is produced by heating white phosphorus under high pressure. It has a cubic structure and is a superconductor at low temperatures.
Each of these allotropes has different physical and chemical properties, making them useful for different applications in science and technology.
What is Required p-Block Elements Group 15 Allotropes of phosphorous
Group 15 elements of the p-block in the periodic table include nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). Among these elements, phosphorus has several allotropes, which are different forms of the same element that exist in the same physical state. The allotropes of phosphorus include white phosphorus, red phosphorus, black phosphorus, violet phosphorus, and Hittorf’s metallic phosphorus.
White phosphorus is a waxy, yellowish-white, highly reactive, and toxic substance that ignites spontaneously in air. It has a tetrahedral molecular structure and is the most reactive form of phosphorus. Red phosphorus is a dark red powder that is less reactive than white phosphorus. It has a polymeric structure and is used in the manufacture of matches, fireworks, and semiconductors. Black phosphorus is a black, crystalline solid that is less reactive than white phosphorus. It has a layered structure and is a semiconductor. Violet phosphorus is a violet or purple-colored solid that is metastable and only exists at high pressures. Hittorf’s metallic phosphorus is a metallic form of phosphorus that is produced by heating white phosphorus under high pressure. It has a cubic structure and is a superconductor at low temperatures.
The different allotropes of phosphorus have different physical and chemical properties, making them useful for various applications in science and technology. For example, white phosphorus is used in the production of pesticides, flame retardants, and fertilizers, while red phosphorus is used in the production of semiconductors, matches, and fireworks. Black phosphorus has potential applications in electronics and optoelectronics, while metallic phosphorus is used in superconductivity research.
When is Required p-Block Elements Group 15 Allotropes of phosphorous
Knowledge about the allotropes of phosphorus in Group 15 p-block elements may be required in various contexts. Some of the possible instances when this knowledge may be required are:
In chemistry classes: Students may learn about the properties and applications of different allotropes of phosphorus as part of their curriculum on p-block elements, inorganic chemistry, or materials science.
In research on materials science and nanotechnology: Researchers may be interested in exploring the properties and potential applications of black phosphorus or other allotropes in the development of new materials for electronic devices, solar cells, or sensors.
In the manufacturing of matches, fireworks, and semiconductors: Industries that produce these products may use red phosphorus as a key ingredient, and understanding its properties is essential for their production.
In the context of environmental health and safety: White phosphorus is a hazardous substance that can cause severe burns and toxicity, and its use and disposal require careful handling and regulation.
Overall, knowledge about the allotropes of phosphorus in Group 15 p-block elements is relevant to various fields, including chemistry, materials science, electronics, and health and safety.
Where is Required p-Block Elements Group 15 Allotropes of phosphorous
Information about the allotropes of phosphorus in Group 15 p-block elements can be found in various resources, including textbooks, scientific journals, and online databases.
In chemistry textbooks, chapters on p-block elements or inorganic chemistry often cover the properties and applications of different allotropes of phosphorus. These textbooks may also provide examples of chemical reactions and physical phenomena that involve phosphorus allotropes.
Scientific journals, such as the Journal of the American Chemical Society, Chemistry of Materials, and Applied Physics Letters, publish research articles on the synthesis, characterization, and applications of phosphorus allotropes. These articles may provide detailed analyses of the chemical and physical properties of different allotropes, as well as insights into their potential uses in various fields.
Online databases, such as the National Institute of Standards and Technology (NIST) Chemistry WebBook, provide information on the thermodynamic, spectroscopic, and transport properties of phosphorus allotropes. These databases may also contain references to relevant research articles and other resources.
In addition, educational websites and online forums related to chemistry, materials science, and nanotechnology may provide accessible explanations of phosphorus allotropes and their properties, as well as examples of their practical applications.
Production of p-Block Elements Group 15 Allotropes of phosphorous
The production of p-block elements Group 15 allotropes of phosphorus involves different methods for each allotrope. Here are some common methods for producing each of the allotropes:
White Phosphorus: White phosphorus is commonly produced by heating phosphate rock with coke and sand in an electric furnace, followed by fractional distillation to purify the product. Alternatively, white phosphorus can also be obtained by reducing phosphates with carbon in a furnace.
Red Phosphorus: Red phosphorus is typically produced by heating white phosphorus in the presence of a reducing agent, such as iodine or hypophosphorous acid, or by reacting white phosphorus with iodine. The process can take place in a sealed reaction vessel or a continuous process furnace.
Black Phosphorus: Black phosphorus can be synthesized using various methods, including chemical vapor deposition (CVD), mechanical exfoliation, and high-pressure techniques. CVD involves depositing phosphorus vapor onto a substrate at high temperatures, while mechanical exfoliation involves peeling off thin layers of black phosphorus from a bulk crystal. High-pressure techniques, such as using a diamond anvil cell, involve subjecting white phosphorus to high pressures above 10 GPa.
Violet Phosphorus: Violet phosphorus is a metastable allotrope that can be synthesized by subjecting white phosphorus to high pressures above 10 GPa. This can be achieved using a diamond anvil cell or other high-pressure techniques.
Hittorf’s Metallic Phosphorus: Hittorf’s metallic phosphorus can be obtained by heating white phosphorus under high pressures above 12 GPa. This can be achieved using a diamond anvil cell or other high-pressure techniques.
Overall, the production of p-block elements Group 15 allotropes of phosphorus requires specialized equipment and expertise, and each allotrope has its unique synthesis methods. The development of new synthesis methods and improved production processes is crucial for advancing the use of these materials in various applications.
How is Required p-Block Elements Group 15 Allotropes of phosphorous
The different allotropes of phosphorus in Group 15 p-block elements have unique chemical and physical properties that arise from variations in their molecular or crystal structures. The following are brief descriptions of how each allotrope of phosphorus differs from one another:
- White phosphorus: This allotrope consists of tetrahedral P4 molecules, where each phosphorus atom is covalently bonded to three other phosphorus atoms. White phosphorus is highly reactive and flammable, readily oxidizing in air and spontaneously igniting in the presence of oxygen or heat.
- Red phosphorus: This allotrope has a polymeric structure, where phosphorus atoms are arranged in long chains with cross-linkages. The polymeric structure makes red phosphorus less reactive than white phosphorus, and it is often used in the production of matches, flame retardants, and semiconductors.
- Black phosphorus: This allotrope has a layered structure, where each layer consists of two-dimensional sheets of covalently bonded phosphorus atoms. Black phosphorus is a semiconductor with a narrow bandgap and high carrier mobility, making it a promising material for electronic and optoelectronic applications.
- Violet phosphorus: This allotrope is metastable and only exists at high pressures above 10 GPa. It has a crystal structure that is intermediate between black phosphorus and white phosphorus.
- Hittorf’s metallic phosphorus: This allotrope is a cubic form of phosphorus that is produced by heating white phosphorus under high pressure. It has a metallic appearance and is a superconductor at low temperatures.
The differences in molecular or crystal structures of these allotropes lead to variations in their reactivity, stability, electronic properties, and other physical characteristics. This makes them useful for different applications in science and technology, such as in the production of semiconductors, electronics, and superconductors.
Case Study on p-Block Elements Group 15 Allotropes of phosphorous
One example of a case study on p-block elements Group 15 allotropes of phosphorus is the development of black phosphorus for electronic and optoelectronic applications.
Black phosphorus has attracted significant attention in recent years due to its unique electronic and optical properties, including a direct bandgap, high carrier mobility, and anisotropic conductivity. These properties make it a promising material for applications such as field-effect transistors, photodetectors, and solar cells.
In a study published in the journal Nano Letters in 2014, researchers from the University of California, Berkeley, and the Lawrence Berkeley National Laboratory synthesized and characterized black phosphorus nanoribbons, which are one-dimensional strips of black phosphorus. The researchers used a simple method of mechanical exfoliation to obtain nanoribbons with controlled widths and lengths, which they then studied using scanning electron microscopy, transmission electron microscopy, and Raman spectroscopy.
The study found that the black phosphorus nanoribbons exhibited high electronic conductivity along the ribbon axis and low conductivity perpendicular to the axis, confirming the anisotropic nature of black phosphorus. The researchers also demonstrated the use of black phosphorus nanoribbons as field-effect transistors, showing that they could achieve high on/off current ratios and low subthreshold swings.
Another study published in the journal Nature Communications in 2017 reported on the use of black phosphorus as a high-performance anode material for lithium-ion batteries. The researchers, from the University of Wollongong in Australia, synthesized black phosphorus nanosheets using a hydrothermal method and tested their electrochemical properties in lithium-ion batteries.
The study found that black phosphorus nanosheets exhibited high reversible capacity, high rate capability, and excellent cycling stability, outperforming other phosphorus-based anode materials such as red phosphorus and white phosphorus. The researchers attributed the superior performance of black phosphorus to its unique two-dimensional structure, which enables fast ion diffusion and high electronic conductivity.
These case studies illustrate the potential of black phosphorus as a versatile and high-performance material for various electronic and energy applications, highlighting the importance of understanding the properties and synthesis methods of different allotropes of phosphorus in p-block elements.
White paper on p-Block Elements Group 15 Allotropes of phosphorous
Introduction:
Phosphorus is a highly reactive non-metal element in Group 15 of the periodic table. It has several allotropes, each with unique chemical and physical properties. In this white paper, we will focus on the p-block elements Group 15 allotropes of phosphorus, including white phosphorus, red phosphorus, black phosphorus, violet phosphorus, and Hittorf’s metallic phosphorus. We will discuss their properties, applications, and synthesis methods.
White Phosphorus:
White phosphorus is the most common allotrope of phosphorus, consisting of tetrahedral P4 molecules. It is a highly reactive and flammable material, readily oxidizing in air and spontaneously igniting in the presence of oxygen or heat. White phosphorus is used in the production of fertilizers, pesticides, and incendiary weapons.
Red Phosphorus:
Red phosphorus has a polymeric structure, where phosphorus atoms are arranged in long chains with cross-linkages. The polymeric structure makes red phosphorus less reactive than white phosphorus, and it is often used in the production of matches, flame retardants, and semiconductors.
Black Phosphorus:
Black phosphorus has a layered structure, where each layer consists of two-dimensional sheets of covalently bonded phosphorus atoms. Black phosphorus is a semiconductor with a narrow bandgap and high carrier mobility, making it a promising material for electronic and optoelectronic applications such as field-effect transistors, photodetectors, and solar cells.
Violet Phosphorus:
Violet phosphorus is a metastable allotrope that exists at high pressures above 10 GPa. It has a crystal structure that is intermediate between black phosphorus and white phosphorus.
Hittorf’s Metallic Phosphorus:
Hittorf’s metallic phosphorus is a cubic form of phosphorus that is produced by heating white phosphorus under high pressure. It has a metallic appearance and is a superconductor at low temperatures.
Applications:
The different allotropes of phosphorus have unique properties that make them useful for various applications. White phosphorus is used in the production of fertilizers, pesticides, and incendiary weapons. Red phosphorus is used in the production of matches, flame retardants, and semiconductors. Black phosphorus is a promising material for electronic and optoelectronic applications such as field-effect transistors, photodetectors, and solar cells. Violet phosphorus is a metastable allotrope that has potential applications in high-pressure physics and materials science. Hittorf’s metallic phosphorus is a superconductor at low temperatures, making it useful for low-temperature electronics and energy applications.
Synthesis Methods:
The different allotropes of phosphorus can be synthesized using various methods. White phosphorus can be obtained from phosphate rocks or by reducing phosphates with carbon in a furnace. Red phosphorus can be obtained by heating white phosphorus in the presence of a reducing agent or by reacting white phosphorus with iodine. Black phosphorus can be synthesized by using chemical vapor deposition, mechanical exfoliation, or high-pressure techniques. Violet phosphorus can be synthesized by subjecting white phosphorus to high pressures above 10 GPa. Hittorf’s metallic phosphorus can be obtained by heating white phosphorus under high pressures above 12 GPa.
Conclusion:
In conclusion, the p-block elements Group 15 allotropes of phosphorus exhibit a wide range of properties and have diverse applications in various fields. White phosphorus is highly reactive and flammable, red phosphorus is less reactive and is used in the production of matches and semiconductors, black phosphorus is a promising material for electronic and optoelectronic applications, violet phosphorus is a metastable allotrope with potential applications in high-pressure physics and materials science, and Hittorf’s metallic phosphorus is a superconductor at low temperatures. The synthesis methods for each allotrope vary, and the development of new synthesis methods is crucial for exploring the full potential of these materials. Overall, the study of p-block elements Group 15 allotropes of phosphorus continues to be an active area of research with promising implications for technology and industry.
