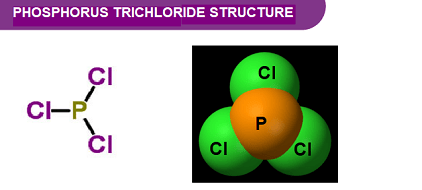
Phosphorus trichloride (PCl3) is a chemical compound that belongs to the group 15 elements in the periodic table, also known as the nitrogen group. It is a colorless, fuming liquid with a pungent odor, and it is highly reactive with water and air.
PCl3 is widely used in the chemical industry as a reagent for the synthesis of a variety of organic and inorganic compounds, including pesticides, pharmaceuticals, and flame retardants. It is also used in the production of plasticizers, dyes, and other industrial chemicals.
In addition, PCl3 is used in the semiconductor industry as a dopant for the production of n-type semiconductors, such as silicon and germanium. It is also used as a catalyst in certain chemical reactions, such as the Friedel-Crafts reaction.
Although PCl3 is widely used, it is a hazardous chemical that should be handled with care. It can cause severe burns upon contact with skin or eyes, and it can release toxic fumes when exposed to water or air. Proper safety precautions, such as the use of protective clothing and equipment, should always be taken when working with PCl3.
What is Required p-Block Elements Group 15 Phosphorus trichloride
Group 15 p-block elements, including phosphorus trichloride, have the outer electronic configuration of ns2np3. This group is also known as the nitrogen group, and it includes nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi).
Phosphorus trichloride is an important member of this group and has several key properties that are characteristic of group 15 elements. These include:
- Trivalent state: Phosphorus trichloride has three valence electrons in its outermost shell, making it a typical trivalent element of the group.
- Reactive with water: Like other group 15 elements, phosphorus trichloride is highly reactive with water, producing hydrochloric acid (HCl) and phosphorous acid (H3PO3).
- Non-metallic nature: Group 15 elements are non-metals with a wide range of properties. Phosphorus trichloride is a non-metallic compound that exists as a liquid at room temperature and atmospheric pressure.
- Lewis acid: Phosphorus trichloride is a Lewis acid, meaning it can accept a pair of electrons from a Lewis base to form a coordinate covalent bond.
- Covalent bonding: Phosphorus trichloride primarily forms covalent bonds due to the electronegativity difference between phosphorus and chlorine.
These properties of phosphorus trichloride make it useful in many industrial and laboratory applications, as well as in the synthesis of a wide range of organic and inorganic compounds.
When is Required p-Block Elements Group 15 Phosphorus trichloride
Group 15 p-block elements, including phosphorus trichloride, are required in various applications across different industries. Some of the specific uses of phosphorus trichloride include:
- Synthesis of organic compounds: Phosphorus trichloride is commonly used as a reagent in organic synthesis, particularly in the conversion of alcohols to alkyl chlorides, and in the preparation of esters and acid chlorides.
- Synthesis of inorganic compounds: Phosphorus trichloride is used in the synthesis of various inorganic compounds, such as phosphorous pentachloride (PCl5), which is a key reagent for the synthesis of phosphoric acid.
- Chemical intermediates: Phosphorus trichloride is an important intermediate for the production of many chemicals, including plasticizers, flame retardants, and surfactants.
- Semiconductors: Phosphorus trichloride is used as a dopant in the semiconductor industry to introduce impurities into silicon and germanium, creating n-type semiconductors.
- Pharmaceuticals: Phosphorus trichloride is used in the synthesis of various pharmaceuticals, such as analgesics and antibiotics.
Overall, phosphorus trichloride is an important chemical that is widely used in the chemical industry for various applications. Its reactivity with water and air, however, makes it a hazardous substance that requires proper handling and safety precautions.
Where is Required p-Block Elements Group 15 Phosphorus trichloride
Phosphorus trichloride, which belongs to the group 15 p-block elements, is required in various industries and applications, including:
- Chemical industry: Phosphorus trichloride is widely used as a reagent in the chemical industry for the synthesis of organic and inorganic compounds, including pesticides, flame retardants, plasticizers, and dyes.
- Semiconductor industry: Phosphorus trichloride is used as a dopant in the semiconductor industry to produce n-type semiconductors, such as silicon and germanium.
- Pharmaceuticals: Phosphorus trichloride is used in the synthesis of various pharmaceuticals, such as analgesics and antibiotics.
- Agriculture: Phosphorus trichloride is used as a pesticide in agriculture to control pests and insects.
- Laboratory research: Phosphorus trichloride is commonly used in laboratory research as a reagent for various chemical reactions.
Phosphorus trichloride is a hazardous substance due to its reactivity with water and air, and therefore requires proper handling and safety precautions. It is usually available from chemical suppliers, and its applications are diverse and widespread across different industries.
How is Required p-Block Elements Group 15 Phosphorus trichloride
Phosphorus trichloride (PCl3), which belongs to the group 15 p-block elements, is primarily produced through the reaction of white phosphorus (P4) with dry chlorine gas (Cl2) at elevated temperatures. The reaction can be represented by the following equation:
P4 + 6 Cl2 → 4 PCl3
The process is typically carried out in a reactor vessel under controlled conditions to ensure high yield and purity of the product. The reaction is highly exothermic, and therefore, it must be carefully controlled to prevent overheating or explosion. Typically, a mixture of PCl3 and unreacted P4 is formed, which is separated by distillation.
Another method for the production of PCl3 is the reaction of phosphorus pentoxide (P2O5) with thionyl chloride (SOCl2), which can be represented by the following equation:
P2O5 + 3 SOCl2 → 2 PCl3 + 3 SO2
This process is carried out at lower temperatures compared to the direct reaction between P4 and Cl2, and it yields a higher purity of PCl3.
Once produced, phosphorus trichloride can be used directly as a reagent in various chemical reactions, or it can be further purified or converted into other derivatives, such as phosphorus oxychloride (POCl3) or phosphorus pentachloride (PCl5), depending on the specific application.
Nomenclature of p-Block Elements Group 15 Phosphorus trichloride
The nomenclature of p-block elements, including phosphorus trichloride, follows the general rules of chemical nomenclature.
The name “phosphorus trichloride” indicates that the compound is made up of phosphorus and chlorine atoms, and that it contains three chlorine atoms per molecule. The prefix “tri-” indicates the number of chlorine atoms, while the element name “phosphorus” is used to indicate the presence of a phosphorus atom.
The formula of phosphorus trichloride is PCl3, where “P” represents the chemical symbol for phosphorus, and “Cl” represents the chemical symbol for chlorine. The subscript “3” indicates the number of chlorine atoms in the molecule.
In addition to the common name, phosphorus trichloride can also be named using the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system. According to this system, the compound is named as “trichloridophosphorus,” where the element name “phosphorus” is followed by the suffix “-id” to indicate that it is an anion, and the prefix “trichlorido-” indicates the number of chlorine atoms in the anion.
Overall, the nomenclature of phosphorus trichloride follows the general rules of chemical nomenclature, and the name or formula can be used to uniquely identify the compound.
Case Study on p-Block Elements Group 15 Phosphorus trichloride
Case Study: Phosphorus Trichloride in the Chemical Industry
Phosphorus trichloride (PCl3) is widely used in the chemical industry for the synthesis of various organic and inorganic compounds. In this case study, we will examine how PCl3 is used in the production of a flame retardant compound and the challenges associated with its use.
Background
Flame retardants are chemical compounds that are added to materials to prevent or delay the spread of fire. One such flame retardant compound is triphenyl phosphate (TPP), which is commonly used in the production of plastics, resins, and textiles.
The synthesis of TPP involves the reaction of phosphorus oxychloride (POCl3) with phenol in the presence of a catalyst. POCl3 is produced by the reaction of PCl3 with oxygen in the air, and therefore, PCl3 is a crucial precursor in the production of TPP.
Challenges
The use of PCl3 in the synthesis of TPP poses several challenges, including safety hazards, environmental concerns, and supply chain issues.
Safety Hazards: PCl3 is a hazardous substance that reacts violently with water and air, producing toxic fumes of hydrogen chloride gas. Therefore, proper handling and storage of PCl3 are crucial to prevent accidents and exposure to workers.
Environmental Concerns: The production and use of PCl3 can generate waste products, such as hydrogen chloride gas and phosphorus waste, which can have harmful effects on the environment. Therefore, proper disposal and treatment of these waste products are necessary to prevent environmental pollution.
Supply Chain Issues: The demand for PCl3 in the chemical industry can fluctuate, causing supply chain issues and price volatility. Moreover, the production of PCl3 requires white phosphorus, which is a limited and non-renewable resource. Therefore, alternative methods for the production of PCl3 are being developed to reduce the reliance on white phosphorus.
Solutions
To address these challenges, various solutions are being implemented, including:
Safety Measures: Proper safety measures are being taken to ensure the safe handling and storage of PCl3. This includes the use of personal protective equipment, ventilation systems, and emergency response procedures.
Environmental Regulations: Environmental regulations are being enforced to ensure the proper disposal and treatment of waste products generated during the production and use of PCl3.
Alternative Production Methods: Alternative methods for the production of PCl3, such as the use of phosphorus pentoxide and thionyl chloride, are being developed to reduce the reliance on white phosphorus and improve the sustainability of the supply chain.
Conclusion
Phosphorus trichloride is a crucial precursor in the chemical industry, especially in the synthesis of flame retardant compounds. However, its use poses various challenges, including safety hazards, environmental concerns, and supply chain issues. To overcome these challenges, proper safety measures, environmental regulations, and alternative production methods are being implemented to ensure the sustainable and safe use of PCl3 in the chemical industry.
White paper on p-Block Elements Group 15 Phosphorus trichloride
White Paper: The Importance of Phosphorus Trichloride in the Chemical Industry
Introduction Phosphorus trichloride (PCl3) is a vital chemical compound that plays a crucial role in the chemical industry. PCl3 is widely used as a reagent and a precursor in the synthesis of various organic and inorganic compounds. In this white paper, we will explore the properties, applications, and production of PCl3 and its significance in the chemical industry.
Properties of Phosphorus Trichloride PCl3 is a colorless to yellowish liquid with a pungent odor. It has a molecular weight of 137.33 g/mol and a boiling point of 76.1°C. PCl3 is soluble in various organic solvents, such as benzene, chloroform, and carbon tetrachloride, but is insoluble in water. The compound has a trigonal pyramidal molecular geometry, with the phosphorus atom at the center and three chlorine atoms attached to it.
Applications of Phosphorus Trichloride PCl3 is a versatile compound that finds numerous applications in the chemical industry. Some of the major applications of PCl3 are:
- Production of flame retardants: PCl3 is a crucial precursor in the synthesis of various flame retardant compounds, such as triphenyl phosphate (TPP) and tris(2-chloroethyl)phosphate (TCEP).
- Production of agrochemicals: PCl3 is used in the synthesis of various agrochemicals, such as insecticides and herbicides.
- Production of pharmaceuticals: PCl3 is a key reagent in the synthesis of various pharmaceuticals, such as sedatives and analgesics.
- Production of plasticizers: PCl3 is used in the production of plasticizers, which are added to plastics to improve their flexibility and durability.
- Production of dyes: PCl3 is used in the production of various dyes, such as acid dyes and azo dyes.
Production of Phosphorus Trichloride PCl3 is primarily produced by the reaction of white phosphorus (P4) with chlorine gas (Cl2) in the presence of a catalyst, such as activated carbon. The reaction is exothermic and produces PCl3 and phosphorus trichloride oxide (POCl3) as the main products. The overall reaction can be represented as:
P4 + 6Cl2 → 4PCl3
POCl3 can be converted to PCl3 by reacting it with hydrogen chloride (HCl) gas, according to the following reaction:
POCl3 + 2HCl → PCl3 + H2O
Significance in the Chemical Industry PCl3 is a vital compound in the chemical industry due to its versatile applications and its use as a precursor in the synthesis of various chemicals. The compound is an essential reagent in the production of flame retardants, which are used in various industries, such as construction, electronics, and automotive. Moreover, PCl3 is also used in the production of agrochemicals, pharmaceuticals, plasticizers, and dyes, which are crucial to many aspects of modern life.
Conclusion
In conclusion, p-Block Elements Group 15 Phosphorus trichloride (PCl3) is a crucial compound in the chemical industry due to its versatile applications and its use as a precursor in the synthesis of various chemicals. PCl3 is used in the production of flame retardants, agrochemicals, pharmaceuticals, plasticizers, and dyes, which are crucial to many aspects of modern life. The production of PCl3 from white phosphorus and chlorine gas is a well-established process. With its unique properties and wide range of applications, PCl3 will continue to be a vital compound in the chemical industry for many years to come.
