Sulfur is a chemical element with the symbol S and atomic number 16. It is a non-metal and can exist in several allotropes, which are different forms of the element that have distinct physical and chemical properties. Here are the main allotropes of sulfur:
- Sulfur (orthorhombic): This is the most stable and common allotrope of sulfur. It consists of cyclic S8 molecules, which are interconnected to form a crystal lattice. This form of sulfur is yellow in color and is insoluble in water.
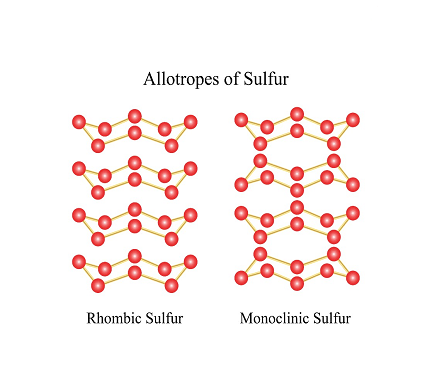
- Sulfur (monoclinic): This is a less stable form of sulfur that can be obtained by cooling molten sulfur rapidly. It consists of long chains of sulfur atoms, which are arranged in a monoclinic crystal structure. This form of sulfur is also yellow in color but is more soluble in organic solvents than the orthorhombic form.
- Plastic sulfur: This form of sulfur is obtained by heating sulfur above its melting point and then cooling it rapidly. It consists of long chains of sulfur atoms, similar to the monoclinic form, but with fewer inter-chain interactions. As a result, plastic sulfur is more flexible and can be molded like plastic. It is also soluble in many organic solvents.
- Rhombic sulfur: This is a metastable form of sulfur that can be obtained by heating the orthorhombic form. It consists of S8 molecules arranged in a rhombic crystal structure. Rhombic sulfur is similar in appearance to the orthorhombic form but has slightly different physical properties.
- Amorphous sulfur: This form of sulfur is not crystalline and does not have a defined structure. It is formed when molten sulfur is rapidly cooled or when sulfur vapor is condensed at low temperatures. Amorphous sulfur is a yellow powder that is often used in the production of sulfuric acid.
These different allotropes of sulfur have different physical and chemical properties and are used in a variety of industrial and scientific applications.
What is Required p-Block Elements Group 16 Allotropes of sulfur
The p-Block elements of Group 16 are Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te) and Polonium (Po).
Sulfur, being a Group 16 element, can exist in several allotropes, which are different forms of the element that have distinct physical and chemical properties. The main allotropes of sulfur include:
- Sulfur (orthorhombic)
- Sulfur (monoclinic)
- Plastic sulfur
- Rhombic sulfur
- Amorphous sulfur
The different allotropes of sulfur have different physical and chemical properties, which make them useful in a variety of industrial and scientific applications. For example, plastic sulfur is more flexible and can be molded like plastic, while the amorphous form is often used in the production of sulfuric acid. Understanding the properties of these different allotropes is important for applications in areas such as materials science, chemical manufacturing, and environmental science.
When is Required p-Block Elements Group 16 Allotropes of sulfur
Knowledge about the Group 16 p-Block elements and their allotropes of sulfur is required in various fields of science and engineering, including:
- Materials science: The different allotropes of sulfur have different physical and chemical properties, which make them useful in the development of new materials. For example, plastic sulfur has been used as an insulating material in electrical components, while the amorphous form is used as a filler in rubber and plastics.
- Chemical manufacturing: Sulfur is an important raw material in the chemical industry, and its different allotropes have different reactivities and properties that make them suitable for different chemical processes. For example, the orthorhombic form is commonly used in the production of sulfuric acid, while the rhombic form is used as a vulcanizing agent in rubber manufacturing.
- Environmental science: Sulfur is an important element in the Earth’s ecosystem, and its different allotropes play a role in natural processes such as the sulfur cycle. Understanding the properties and behavior of these different allotropes can help in developing strategies to mitigate the environmental impacts of sulfur pollution.
Overall, knowledge about the Group 16 p-Block elements and their allotropes of sulfur is important in many scientific and engineering fields and has applications in various industries, including materials science, chemical manufacturing, and environmental science.
Where is Required p-Block Elements Group 16 Allotropes of sulfur
Knowledge about the Group 16 p-Block elements and their allotropes of sulfur is required in various fields of science and engineering, and is taught in different educational institutions, including universities, colleges, and high schools, in courses such as chemistry, materials science, and environmental science.
In addition, research institutions and industries involved in the development of new materials, chemical processes, and environmental technologies also require a deep understanding of the properties and behavior of sulfur and its allotropes, making this knowledge relevant in the workplace for scientists, engineers, and technicians.
Overall, the knowledge of Group 16 p-Block elements and their allotropes of sulfur is important both in academic and professional settings, and can be found in various educational resources, scientific journals, and technical publications.
How is Required p-Block Elements Group 16 Allotropes of sulfur
The properties and behavior of Group 16 p-Block elements and their allotropes of sulfur are studied using various experimental and theoretical methods.
Experimental methods include techniques such as X-ray diffraction, infrared spectroscopy, and thermal analysis to determine the crystal structure, chemical bonding, and thermal stability of sulfur allotropes. Chemical reactions involving sulfur are also studied to understand the reactivity and chemical properties of different allotropes.
Theoretical methods such as quantum chemistry and molecular dynamics simulations are used to model the behavior of sulfur allotropes at the atomic and molecular level, providing insight into their electronic structure, chemical reactivity, and physical properties.
Overall, the study of Group 16 p-Block elements and their allotropes of sulfur involves a combination of experimental and theoretical approaches, providing a comprehensive understanding of the properties and behavior of these elements and their potential applications in various scientific and engineering fields.
Nomenclature of p-Block Elements Group 16 Allotropes of sulfur
The nomenclature of the p-Block Elements Group 16 Allotropes of sulfur is based on the crystal structure of each allotrope. The main allotropes of sulfur include:
- Orthorhombic sulfur: This allotrope has an orthorhombic crystal structure and is also known as alpha-sulfur. Its chemical formula is S8.
- Monoclinic sulfur: This allotrope has a monoclinic crystal structure and is also known as beta-sulfur. Its chemical formula is also S8.
- Plastic sulfur: This allotrope has a highly disordered and amorphous structure, and does not have a defined chemical formula.
- Rhombic sulfur: This allotrope has a rhombic crystal structure and is also known as gamma-sulfur. Its chemical formula is S8.
- Amorphous sulfur: This allotrope has a highly disordered and amorphous structure, and does not have a defined chemical formula.
In general, the nomenclature of sulfur allotropes is based on their crystal structure and not their chemical composition, as most sulfur allotropes have the same chemical formula of S8. However, some amorphous forms of sulfur may have varying degrees of disorder and a less defined composition, making their nomenclature more difficult.
Case Study on p-Block Elements Group 16 Allotropes of sulfur
One interesting case study related to p-Block Elements Group 16 Allotropes of sulfur is their use in the development of new cathode materials for rechargeable batteries. Sulfur has a high theoretical capacity as a cathode material, which means it can store a large amount of energy per unit of weight, making it an attractive candidate for use in rechargeable batteries.
However, one of the main challenges of using sulfur as a cathode material is its low conductivity, which can limit the battery’s performance. To address this challenge, researchers have developed different sulfur allotropes and modified their properties to improve their performance as cathode materials.
One approach is to use a hybrid structure of sulfur with a conductive material such as carbon to improve its conductivity. In this approach, sulfur is incorporated into the carbon matrix to form a composite material, which maintains the high energy storage capacity of sulfur while also improving its conductivity.
Another approach is to use a nanostructured form of sulfur, such as sulfur nanoparticles or sulfur nanotubes, which have a high surface area and can improve the battery’s performance by increasing the electrode’s reactivity and reducing the diffusion path length.
Overall, the development of new sulfur allotropes and their modification for use as cathode materials in rechargeable batteries is an active area of research, with potential applications in various fields such as portable electronics, electric vehicles, and renewable energy storage.
White paper on p-Block Elements Group 16 Allotropes of sulfur
Introduction:
The p-Block Elements Group 16 Allotropes of sulfur are a group of chemical elements that have a wide range of applications in various fields of science and engineering. The group includes six elements: oxygen, sulfur, selenium, tellurium, polonium, and livermorium, all of which have six valence electrons and exhibit similar chemical properties. In particular, sulfur is one of the most widely used elements in the chemical industry, with applications in the production of fertilizers, detergents, and pharmaceuticals, among others. Moreover, the various allotropes of sulfur have unique physical and chemical properties, making them useful for various applications.
Properties of Group 16 p-Block Elements:
The Group 16 p-Block Elements have unique physical and chemical properties that make them useful in various applications. For example, oxygen is a highly reactive gas that is essential for combustion and respiration, while sulfur is a solid that exists in a variety of allotropes and is used in the production of sulfuric acid, fertilizers, and detergents. Selenium and tellurium are both semiconductors and have applications in electronics and photovoltaics, while polonium and livermorium are highly radioactive elements that have applications in nuclear science and technology.
Allotropes of Sulfur:
Sulfur has several allotropes, including orthorhombic, monoclinic, plastic, rhombic, and amorphous sulfur. Each allotrope has a unique crystal structure and physical properties that make it useful for various applications. For example, rhombic sulfur is used as a vulcanizing agent for rubber, while plastic sulfur is used as a precursor for making other sulfur compounds. Orthorhombic sulfur is the most stable form of sulfur and is used in the production of sulfuric acid, while amorphous sulfur is used as a fungicide and pesticide.
Applications of Group 16 p-Block Elements:
The Group 16 p-Block Elements and their allotropes have a wide range of applications in various fields. For example, oxygen is essential for combustion and respiration, while sulfur is used in the production of sulfuric acid, fertilizers, and detergents. Selenium and tellurium have applications in electronics and photovoltaics, while polonium and livermorium have applications in nuclear science and technology. Moreover, the various allotropes of sulfur have unique physical and chemical properties that make them useful for various applications, such as vulcanizing rubber, making other sulfur compounds, and as a fungicide and pesticide.
Conclusion:
The p-Block Elements Group 16 Allotropes of sulfur are a group of chemical elements with unique physical and chemical properties that make them useful for various applications. The various allotropes of sulfur have unique properties that make them useful in various applications, such as vulcanizing rubber, making other sulfur compounds, and as a fungicide and pesticide. The Group 16 p-Block Elements have applications in various fields, such as the chemical industry, electronics, photovoltaics, and nuclear science and technology. Overall, the knowledge of Group 16 p-Block Elements and their allotropes is essential for various scientific and engineering fields and can be applied to develop new materials, chemical processes, and environmental technologies.
