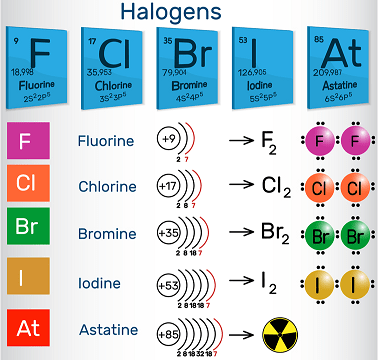
The incandescent lamp (/ˈhælədʒən, ˈheɪ-, – loʊ-, – ˌdʒɛn/) are a gathering in the occasional table comprising of six synthetically related components: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts), however some authors[who?] would reject tennessine as its science is obscure and is hypothetically expected to be more similar to that of gallium. In the advanced IUPAC terminology, this gathering is known as gathering 17.
“Halogen” signifies “salt previous” or “salt producer”. At the point when incandescent lamp respond with metals, they produce a large number of salts, including calcium fluoride, sodium chloride (normal table salt), silver bromide and potassium iodide.
The gathering of incandescent lamp is the main occasional table gathering that contains components in three of the fundamental conditions of issue at standard temperature and tension, however not far above room temperature the equivalent turns out to be valid for bunches 1 and 15, accepting white phosphorus is taken as the standard state. Every one of the incandescent lamp structure acids when attached to hydrogen. Most incandescent light are regularly delivered from minerals or salts. The center incandescent light — chlorine, bromine, and iodine — are frequently utilized as sanitizers. Organobromides are the main class of fire retardants, while essential incandescent light are hazardous and can be harmful.
What is Required p-Block Elements Group 16 Halogen
The group 16 halogens have several important properties and applications. Some of the key characteristics and uses of the group 16 halogens are as follows:
- Reactivity: Halogens are highly reactive non-metals, and they readily react with other elements to form compounds. This reactivity is due to the presence of seven valence electrons in their outermost shell.
- Oxidation state: The halogens have a wide range of oxidation states, but they most commonly exist in the -1 oxidation state.
- Electron affinity: Halogens have a high electron affinity, which means they have a strong tendency to gain an electron and become negatively charged ions.
- Electronegativity: The halogens are highly electronegative, which means they have a strong tendency to attract electrons towards themselves when forming chemical bonds.
- Applications: Halogens have many important applications, including use as disinfectants, bleaching agents, and in the production of plastics, pesticides, and pharmaceuticals.
Some specific examples of the applications of group 16 halogens are:
- Chlorine is used to disinfect water and swimming pools, and is also used in the production of PVC plastics and other industrial chemicals.
- Bromine is used as a flame retardant in plastics and textiles, and is also used in the production of pharmaceuticals and agricultural chemicals.
- Iodine is used in the production of iodized salt, as well as in pharmaceuticals and photographic chemicals.
When is Required p-Block Elements Group 16 Halogen
Group 16 halogens are required or useful in various contexts, including in chemistry, industry, and everyday life. Some specific examples of when group 16 halogens are required are:
- Water treatment: Chlorine is commonly used to disinfect water in water treatment plants, helping to eliminate harmful microorganisms and bacteria.
- Industrial processes: Halogens such as chlorine and bromine are used in the production of a wide range of industrial chemicals, including plastics, pesticides, and pharmaceuticals.
- Photography: Iodine is used in the production of photographic chemicals, including silver iodide, which is used to create light-sensitive photographic emulsions.
- Health: Iodine is required for the synthesis of thyroid hormones in the body, which are important for regulating metabolism and growth.
- Food industry: Halogens are used as food preservatives, such as potassium iodide which is added to salt in order to prevent iodine deficiency in populations.
Overall, the unique properties of group 16 halogens make them useful in a wide range of applications, from water treatment and industrial processes to health and food industry.
Where is Required p-Block Elements Group 16 Halogen
Group 16 halogens can be found in various locations and contexts, including:
- Earth’s crust: Halogens are abundant in the Earth’s crust, and are found in various minerals and ores. For example, iodine is found in seawater and in some mineral deposits, while sulfur is found in sulfide and sulfate minerals.
- Laboratory: Halogens are commonly used in laboratory settings for a wide range of experiments and applications, including in analytical chemistry, organic synthesis, and materials science.
- Industry: Halogens are used extensively in the industrial production of chemicals, plastics, and other materials. Chlorine, for example, is used in the production of PVC plastics, while bromine is used as a flame retardant in textiles and plastics.
- Water treatment plants: Halogens such as chlorine are used in water treatment plants to disinfect water and eliminate harmful microorganisms.
- Health: Halogens such as iodine are essential for human health, as they are required for the synthesis of thyroid hormones in the body. Iodine deficiency can lead to serious health problems, including goiter and cretinism.
Overall, group 16 halogens can be found in a wide range of locations and contexts, from the Earth’s crust to industrial and laboratory settings, and even in the human body.
How is Required p-Block Elements Group 16 Halogen
The properties and behavior of group 16 halogens can be described in various ways. Here are some of the ways in which group 16 halogens are typically characterized:
- Electronic configuration: Group 16 halogens have six valence electrons in their outermost shell, which makes them highly reactive and prone to forming chemical bonds.
- Reactivity: Group 16 halogens are highly reactive nonmetals, and they readily form compounds with other elements. They have a high electron affinity, meaning they readily accept electrons to form negatively charged ions. They are also highly electronegative, meaning they attract electrons towards themselves when forming chemical bonds.
- Oxidation state: Group 16 halogens can exhibit a wide range of oxidation states, but they most commonly exist in the -1 oxidation state.
- Physical properties: Group 16 halogens have relatively low melting and boiling points, and they exist as diatomic molecules in their elemental form. They are also relatively poor conductors of heat and electricity.
- Applications: Group 16 halogens have a wide range of applications, including in water treatment, industrial production, health, and food industry.
Overall, the properties and behavior of group 16 halogens are characterized by their high reactivity, ability to form chemical bonds, and their importance in various industrial, scientific, and everyday applications.
Nomenclature of p-Block Elements Group 16 Halogen
The nomenclature of group 16 halogens follows the standard naming conventions for chemical elements. The elements are named based on their atomic number, which indicates the number of protons in the nucleus of each atom. Here are the names and symbols for the group 16 halogens:
- Oxygen: O
- Sulfur: S
- Selenium: Se
- Tellurium: Te
- Polonium: Po
Note that the first four elements (oxygen, sulfur, selenium, and tellurium) are nonmetals, while polonium is a metalloid.
In addition to their standard names and symbols, group 16 halogens can also be referred to using their periodic table group number, which is 16. For example, sulfur can be referred to as “group 16 element” or “group 16 chalcogen.”
It is also worth noting that the halogens in general (including group 16 halogens) are sometimes referred to as the “salt formers,” due to their high reactivity and tendency to form salts with metals.
Case Study on p-Block Elements Group 16 Halogen
One notable case study on group 16 halogens involves the use of iodine-131 in medical imaging and treatment. Iodine-131 is a radioactive isotope of iodine that is commonly used in nuclear medicine to diagnose and treat thyroid disorders, such as hyperthyroidism and thyroid cancer.
Iodine-131 is produced by bombarding stable iodine-127 with neutrons, which converts some of the iodine-127 into iodine-131. The resulting iodine-131 isotope has a half-life of approximately 8 days, which means it decays rapidly over time.
In medical imaging, iodine-131 is used in a procedure called a radioactive iodine uptake (RAIU) test. In this test, a small amount of iodine-131 is ingested or injected into the patient’s body, and then the amount of iodine absorbed by the thyroid gland is measured using a special scanner. This can help diagnose thyroid disorders, such as hyperthyroidism or thyroid cancer.
In medical treatment, iodine-131 is used to selectively destroy thyroid tissue in patients with hyperthyroidism or thyroid cancer. The iodine-131 is taken up by the thyroid gland, where its radioactive emissions can damage or destroy the thyroid tissue. This can help reduce the production of thyroid hormones, and can also help kill cancer cells in patients with thyroid cancer.
While iodine-131 has proven to be an effective tool in medical imaging and treatment, it also poses some risks due to its radioactive nature. Patients receiving iodine-131 treatment must be carefully monitored for radiation exposure, and precautions must be taken to minimize the risk of contamination to others.
In conclusion, iodine-131 is a notable case study on group 16 halogens and their use in medical imaging and treatment. Its unique properties and behavior make it an effective tool for diagnosing and treating thyroid disorders, but also require careful management to minimize the risk of radiation exposure.
White paper on p-Block Elements Group 16 Halogen
Title: Group 16 Halogens: Properties, Applications, and Future Prospects
Abstract:
Group 16 halogens are a group of nonmetallic chemical elements that include oxygen, sulfur, selenium, tellurium, and polonium. These elements exhibit unique properties and behaviors that make them essential to various industrial, scientific, and everyday applications. In this white paper, we provide an overview of the properties and applications of group 16 halogens, and discuss some of the future prospects and challenges associated with these elements.
Introduction:
Group 16 halogens are located in the p-block of the periodic table and share several common characteristics, such as having six valence electrons and a tendency to form -1 anions. These elements are widely used in a variety of applications, including chemical synthesis, industrial production, and biological processes. For example, sulfur is used in the production of fertilizers, rubber, and dyes, while selenium is used in electronic devices, photovoltaic cells, and nutritional supplements.
Properties of Group 16 Halogens:
Group 16 halogens have several unique properties that distinguish them from other elements. For example, they have relatively low melting and boiling points, and they exist as diatomic molecules in their elemental form. They also exhibit high electron affinity and electronegativity, meaning they readily accept or attract electrons when forming chemical bonds. In addition, they can exhibit a wide range of oxidation states, with -1 being the most common.
Applications of Group 16 Halogens:
Group 16 halogens have a wide range of applications in various industries and scientific fields. Some of the most common applications include:
- Chemical synthesis: Group 16 halogens are used in the production of a variety of chemical compounds, including fertilizers, pigments, and pharmaceuticals.
- Industrial production: Sulfur is used in the production of rubber, paper, and other industrial materials, while selenium is used in electronic devices and photovoltaic cells.
- Biological processes: Group 16 halogens are essential to many biological processes, such as oxygen transport in hemoglobin and enzyme catalysis.
- Nuclear medicine: Iodine-131, a radioactive isotope of iodine, is used in medical imaging and treatment of thyroid disorders.
Future Prospects and Challenges:
As the demand for renewable energy sources and sustainable technologies continues to grow, group 16 halogens are poised to play an increasingly important role in these industries. For example, selenium is being explored as a potential material for next-generation photovoltaic cells, while tellurium is used in the production of high-efficiency solar cells. However, the supply of these elements is limited, and there are concerns about the environmental impact of their extraction and production. To overcome these challenges, there is a need for increased research and development of sustainable technologies for the extraction and production of group 16 halogens.
Conclusion:
Group 16 halogens are a group of nonmetallic chemical elements with unique properties and behaviors that make them essential to various industrial, scientific, and everyday applications. They have a wide range of applications, including chemical synthesis, industrial production, and biological processes. As the demand for sustainable technologies continues to grow, group 16 halogens are poised to play an increasingly important role in these industries. However, there are also challenges associated with their extraction and production, and there is a need for increased research and development of sustainable technologies to address these challenges.
