The Group 17 halogens, also known as the halides, form a series of oxoacids with varying numbers of oxygen atoms. The oxoacids of the halogens are named based on the number of oxygen atoms in the molecule and the oxidation state of the halogen.
Here are the oxoacids of the halogens:
- Fluorine: There are no known oxoacids of fluorine.
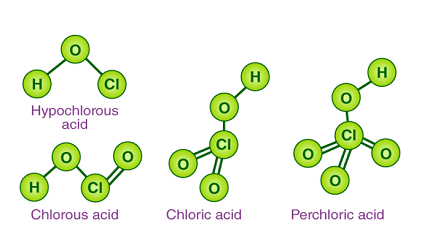
- Chlorine: Chlorine forms four oxoacids: hypochlorous acid (HOCl), chlorous acid (HClO2), chloric acid (HClO3), and perchloric acid (HClO4).
- Bromine: Bromine forms five oxoacids: hypobromous acid (HOBr), bromous acid (HBrO2), bromic acid (HBrO3), perbromic acid (HBrO4), and perbromous acid (HBrO).
- Iodine: Iodine forms seven oxoacids: hypoiodous acid (HOI), iodous acid (HIO2), iodic acid (HIO3), periodate (HIO4), periodic acid (H5IO6), perioditite (H4IO6), and per-iodic acid (HIO6).
The oxoacids of the halogens have different chemical properties and uses. For example, hypochlorous acid is a powerful disinfectant, while perchloric acid is used as a laboratory reagent.
What is Required p-Block Elements Group 17 Oxoacids of halogens
The p-Block Elements Group 17 Oxoacids of halogens are a part of the chemistry curriculum of high school and undergraduate level. Students are usually required to learn the names, formulas, and properties of the oxoacids of the halogens.
The group 17 halogens are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements form a series of oxoacids with varying numbers of oxygen atoms. The oxoacids of the halogens have different chemical properties and uses, which are important to understand.
The required knowledge about the p-Block Elements Group 17 Oxoacids of halogens may include:
- The names and formulas of the oxoacids of the halogens.
- The oxidation states of the halogens in each oxoacid.
- The structures and shapes of the oxoacids.
- The properties and uses of the oxoacids, including acidity, oxidizing power, and reactivity with other substances.
- The relationships between the number of oxygen atoms in the oxoacid and its properties, such as acidity and stability.
A good understanding of the p-Block Elements Group 17 Oxoacids of halogens is important for students who are pursuing a career in chemistry or related fields. It provides a foundation for further study of the chemistry of the halogens and their compounds.
When is Required p-Block Elements Group 17 Oxoacids of halogens
The Required p-Block Elements Group 17 Oxoacids of halogens are typically covered in high school and undergraduate level chemistry courses. In most chemistry curriculums, the study of the p-Block Elements, including Group 17, is usually covered after the study of the basic principles of chemistry, such as atomic structure, chemical bonding, and chemical reactions.
The study of the p-Block Elements, including Group 17, is an important part of chemistry education as it provides a foundation for understanding the properties and reactivity of a wide range of chemical substances. The study of the oxoacids of the halogens is particularly important as these compounds have a wide range of uses and applications, including as disinfectants, laboratory reagents, and oxidizing agents.
In high school level chemistry, the Required p-Block Elements Group 17 Oxoacids of halogens are usually covered in the section on the properties of the halogens, along with their other compounds such as halides, oxyhalides, and interhalogens.
In undergraduate level chemistry, the Required p-Block Elements Group 17 Oxoacids of halogens are usually covered in more detail, along with other aspects of the chemistry of the halogens, such as their reactions with other elements, their physical properties, and their chemical bonding.
Where is Required p-Block Elements Group 17 Oxoacids of halogens
The Required p-Block Elements Group 17 Oxoacids of halogens are typically studied in chemistry courses, both in high school and at the undergraduate level. These courses are usually offered in educational institutions such as high schools, colleges, and universities.
In high school, chemistry courses are typically required for students who are pursuing a career in science or engineering. In these courses, the study of the p-Block Elements, including Group 17, is usually covered as part of the chemistry curriculum.
In undergraduate level chemistry, the Required p-Block Elements Group 17 Oxoacids of halogens are typically covered in courses such as general chemistry, inorganic chemistry, and physical chemistry. These courses are typically offered in colleges and universities.
The Required p-Block Elements Group 17 Oxoacids of halogens can also be found in chemistry textbooks, reference books, and online resources. These resources provide detailed information on the properties and reactivity of the oxoacids of the halogens, as well as their uses and applications.
Overall, the Required p-Block Elements Group 17 Oxoacids of halogens can be found in a variety of educational settings, including schools, colleges, and universities, as well as in textbooks and online resources.
How is Required p-Block Elements Group 17 Oxoacids of halogens
The Required p-Block Elements Group 17 Oxoacids of halogens are typically studied through a combination of classroom lectures, laboratory experiments, and independent study.
Classroom lectures provide an overview of the properties and reactivity of the oxoacids of the halogens, as well as their uses and applications. In these lectures, instructors typically cover topics such as the names and formulas of the oxoacids, their structures and shapes, their acidity and oxidizing power, and the relationship between the number of oxygen atoms and their properties.
Laboratory experiments provide students with hands-on experience in working with the oxoacids of the halogens. In these experiments, students learn how to prepare and handle the oxoacids safely, and how to analyze their properties using techniques such as titration and spectroscopy.
Independent study is also an important component of learning about the Required p-Block Elements Group 17 Oxoacids of halogens. This can involve reading textbooks and reference books on the subject, working through practice problems and exercises, and using online resources such as videos and interactive simulations.
Overall, learning about the Required p-Block Elements Group 17 Oxoacids of halogens involves a combination of different teaching methods and resources, and requires students to be actively engaged in their learning through both classroom instruction and independent study.
Nomenclature of p-Block Elements Group 17 Oxoacids of halogens
The nomenclature of p-Block Elements Group 17 Oxoacids of halogens follows a general pattern. The name of the oxoacid is based on the name of the halogen, with the suffix “-ic” or “-ous” added to indicate the oxidation state of the halogen. The number of oxygen atoms in the oxoacid is indicated by a prefix such as “per-“, “hypo-“, or by using suffixes such as “-ate” or “-ite”. Here are some examples of the nomenclature of p-Block Elements Group 17 Oxoacids of halogens:
- Chloric acid (HClO3): This oxoacid contains chlorine in the +5 oxidation state and has three oxygen atoms. The suffix “-ic” indicates the higher oxidation state of chlorine.
- Hypochlorous acid (HClO): This oxoacid contains chlorine in the +1 oxidation state and has one oxygen atom. The prefix “hypo-” indicates the lowest oxidation state of chlorine.
- Perchloric acid (HClO4): This oxoacid contains chlorine in the +7 oxidation state and has four oxygen atoms. The prefix “per-” indicates the highest oxidation state of chlorine.
- Chlorous acid (HClO2): This oxoacid contains chlorine in the +3 oxidation state and has two oxygen atoms. The suffix “-ous” indicates the lower oxidation state of chlorine.
- Perbromic acid (HBrO4): This oxoacid contains bromine in the +7 oxidation state and has four oxygen atoms. The prefix “per-” indicates the highest oxidation state of bromine.
- Iodic acid (HIO3): This oxoacid contains iodine in the +5 oxidation state and has three oxygen atoms. The suffix “-ic” indicates the higher oxidation state of iodine.
In general, the nomenclature of p-Block Elements Group 17 Oxoacids of halogens follows a consistent pattern based on the oxidation state and number of oxygen atoms in the compound, making it relatively easy to predict the name of a given oxoacid.
Case Study on p-Block Elements Group 17 Oxoacids of halogens
One example of the use of p-Block Elements Group 17 Oxoacids of halogens is in the treatment of water. Chlorine is commonly used to disinfect water and kill harmful bacteria, viruses, and other microorganisms that may be present. This is typically done by adding chlorine gas or a chlorine-containing compound such as sodium hypochlorite to the water.
However, chlorine can also react with organic matter in the water to form potentially harmful byproducts such as trihalomethanes (THMs). These byproducts have been linked to a variety of health problems, including cancer and reproductive issues.
To address this issue, p-Block Elements Group 17 Oxoacids of halogens such as chloramines (compounds containing both chlorine and ammonia) have been developed as an alternative to chlorine for water treatment. Chloramines are less reactive with organic matter than chlorine, so they produce fewer THMs and other harmful byproducts.
In addition, chloramines are more stable than chlorine, so they can provide longer-lasting disinfection. This makes them particularly useful in large water treatment facilities, where it may be difficult to maintain a consistent level of chlorine throughout the entire water distribution system.
However, there are some potential drawbacks to the use of chloramines. For example, they may be less effective than chlorine at killing certain types of bacteria, viruses, and other microorganisms. In addition, they can also produce their own set of harmful byproducts, although these are typically less toxic than THMs.
Overall, the use of p-Block Elements Group 17 Oxoacids of halogens such as chloramines represents an important application of these compounds in the field of water treatment. By providing an alternative to chlorine, these compounds can help to reduce the formation of potentially harmful byproducts while still providing effective disinfection of water.
White paper on p-Block Elements Group 17 Oxoacids of halogens
Introduction: The p-Block Elements Group 17 Oxoacids of halogens are a group of compounds that are important in many different fields, including chemistry, medicine, and industry. These compounds are characterized by the presence of a halogen atom (fluorine, chlorine, bromine, iodine, or astatine) bonded to one or more oxygen atoms, with the resulting compounds having different oxidation states and properties.
In this white paper, we will discuss the properties, applications, and potential future directions for p-Block Elements Group 17 Oxoacids of halogens.
Properties: The properties of p-Block Elements Group 17 Oxoacids of halogens vary depending on the specific compound, but they all share certain common characteristics. Halogens are highly electronegative elements, meaning that they have a strong attraction for electrons. This makes them highly reactive and able to form strong covalent bonds with other elements.
In addition, the electronegativity of halogens allows them to form a variety of oxidation states, ranging from -1 to +7. This property is important for the formation of oxoacids, which are characterized by the presence of oxygen atoms and different oxidation states of the halogen atom.
Applications: The p-Block Elements Group 17 Oxoacids of halogens have a wide range of applications in various fields.
- Water treatment: Chlorine and other halogens are commonly used to disinfect water, but they can also produce harmful byproducts such as trihalomethanes (THMs). To address this issue, p-Block Elements Group 17 Oxoacids of halogens such as chloramines have been developed as an alternative to chlorine for water treatment. Chloramines are less reactive with organic matter than chlorine, so they produce fewer THMs and other harmful byproducts.
- Medicine: Halogens are important elements in many pharmaceutical compounds. For example, iodine is commonly used as a disinfectant and as a component of contrast agents for medical imaging. Bromine is used in the treatment of certain types of cancer, while fluorine is used in the development of many different types of drugs, including antibiotics and anti-cancer agents.
- Industry: Halogens are used in a variety of industrial processes, including the production of plastics, solvents, and pesticides. Chlorine is used in the production of PVC and other plastics, while bromine is used in the production of flame retardants.
Future Directions: There are several potential future directions for research on p-Block Elements Group 17 Oxoacids of halogens.
- Green chemistry: Many industrial processes that use halogens are associated with high energy consumption and the production of hazardous waste. Research is underway to develop more sustainable and environmentally friendly alternatives that use p-Block Elements Group 17 Oxoacids of halogens.
- Drug development: The unique properties of halogens make them valuable components of many different types of drugs. Research is underway to develop new drugs that incorporate p-Block Elements Group 17 Oxoacids of halogens in novel ways.
- Water treatment: While chloramines are less reactive than chlorine and produce fewer harmful byproducts, they are also less effective at killing certain types of microorganisms. Research is underway to develop new p-Block Elements Group 17 Oxoacids of halogens that provide effective disinfection while minimizing the production of harmful byproducts.
Conclusion:
In conclusion, p-Block Elements Group 17 Oxoacids of halogens are a group of compounds that are important in many different fields. They are characterized by the presence of a halogen atom bonded to one or more oxygen atoms, resulting in compounds with different oxidation states and properties. The electronegativity of halogens allows them to form a variety of oxidation states, which is important for the formation of oxoacids.
These compounds have a wide range of applications, including water treatment, medicine, and industry. Ongoing research is focused on developing more sustainable and environmentally friendly industrial processes, new drugs, and improving water treatment methods. The unique properties of halogens make them valuable components of many different types of compounds and materials, and continued research into these compounds will likely lead to even more applications in the future.
