Group 17 in the periodic table contains the halogens, including chlorine. Chlorine has many uses, some of which are:
- Water disinfection: Chlorine is commonly used to disinfect drinking water and swimming pools, as it is effective in killing bacteria and viruses.
- Bleaching agent: Chlorine is used as a bleaching agent in the manufacturing of paper, textiles, and many other products.
- PVC production: Chlorine is used to produce polyvinyl chloride (PVC), a widely used plastic material.
- Solvent: Chlorine is used as a solvent for a variety of organic compounds.
- Pharmaceuticals: Chlorine is used in the production of pharmaceuticals, including antibiotics and antiseptics.
- Cleaning agent: Chlorine is used in cleaning products, such as bleach and disinfectant sprays.
- Pesticides: Chlorine is used in the production of many pesticides and herbicides.
- Refrigeration: Chlorine is used in the production of refrigerants, such as chlorofluorocarbons (CFCs), which are now being phased out due to their impact on the ozone layer.
- Flame retardants: Chlorine is used in the production of flame retardants for textiles and plastics.
- Metal refining: Chlorine is used in the refining of metals, such as copper and nickel, by removing impurities.
What is Required p-Block Elements Group 17 Uses of chlorine
The p-Block elements in Group 17 of the periodic table are known as the halogens, and include chlorine. The uses of chlorine are as follows:
- Water purification: Chlorine is used for the purification of water. It is added to drinking water and swimming pools to kill bacteria and other microorganisms.
- Disinfectant: Chlorine is a powerful disinfectant and is used in hospitals and other healthcare facilities to sanitize equipment and surfaces.
- Bleaching agent: Chlorine is used as a bleaching agent in the manufacturing of paper, textiles, and other products.
- PVC production: Chlorine is used in the production of polyvinyl chloride (PVC), a widely used plastic material.
- Solvent: Chlorine is used as a solvent for many organic compounds.
- Pharmaceuticals: Chlorine is used in the production of pharmaceuticals, including antibiotics and antiseptics.
- Cleaning agent: Chlorine is used in cleaning products, such as bleach and disinfectant sprays.
- Pesticides: Chlorine is used in the production of many pesticides and herbicides.
- Refrigeration: Chlorine is used in the production of refrigerants, such as chlorofluorocarbons (CFCs), which are now being phased out due to their impact on the ozone layer.
- Flame retardants: Chlorine is used in the production of flame retardants for textiles and plastics.
Overall, the uses of chlorine are vast and varied, making it an important element in many industries.
Where is Required p-Block Elements Group 17 Uses of chlorine
Chlorine, which is a member of Group 17 of the periodic table, has many uses across various industries around the world. Some common places where chlorine is used include:
- Water treatment plants: Chlorine is added to drinking water and swimming pools to kill bacteria and other microorganisms.
- Hospitals and healthcare facilities: Chlorine is used as a disinfectant to sanitize equipment and surfaces.
- Manufacturing facilities: Chlorine is used in the manufacturing of paper, textiles, and other products as a bleaching agent.
- PVC production facilities: Chlorine is used in the production of polyvinyl chloride (PVC), a widely used plastic material.
- Laboratories: Chlorine is used as a solvent for many organic compounds in laboratories.
- Pharmaceuticals manufacturing facilities: Chlorine is used in the production of pharmaceuticals, including antibiotics and antiseptics.
- Household cleaning: Chlorine is used in cleaning products, such as bleach and disinfectant sprays, that are used in homes.
- Agricultural fields: Chlorine is used in the production of many pesticides and herbicides that are used to protect crops.
- Industrial refrigeration: Chlorine is used in the production of refrigerants, such as chlorofluorocarbons (CFCs), which were widely used in industrial refrigeration systems before they were phased out due to their impact on the ozone layer.
- Textile and plastic manufacturing facilities: Chlorine is used in the production of flame retardants for textiles and plastics.
Overall, the uses of chlorine are widespread and can be found in many places across various industries around the world.
How is Required p-Block Elements Group 17 Uses of chlorine
Chlorine, which is a member of Group 17 of the periodic table, is used in a variety of ways across different industries. The specific methods of using chlorine can vary depending on the application, but some common ways in which chlorine is used include:
- Water purification: Chlorine is added to water treatment plants and swimming pools to kill bacteria and other microorganisms. The amount of chlorine added depends on the volume of water being treated and the level of contamination.
- Disinfectant: Chlorine is used as a disinfectant in healthcare facilities to sanitize equipment and surfaces. Chlorine-based disinfectants are applied to surfaces and left for a specified period of time to kill any pathogens present.
- Bleaching agent: Chlorine is used as a bleaching agent in the manufacturing of paper, textiles, and other products. The process involves treating the material with a solution of chlorine or a chlorine compound to remove any color or impurities.
- PVC production: Chlorine is used in the production of polyvinyl chloride (PVC), a widely used plastic material. The process involves reacting chlorine gas with ethylene to produce vinyl chloride, which is then polymerized to form PVC.
- Solvent: Chlorine is used as a solvent for many organic compounds. In this application, chlorine is often used as part of a larger solvent mixture to dissolve and extract certain compounds.
- Pharmaceuticals: Chlorine is used in the production of pharmaceuticals, including antibiotics and antiseptics. The specific process for using chlorine in pharmaceutical production can vary depending on the compound being produced.
- Cleaning agent: Chlorine is used in cleaning products, such as bleach and disinfectant sprays. These products are typically applied to surfaces and left for a specified period of time to kill pathogens and remove stains.
- Pesticides: Chlorine is used in the production of many pesticides and herbicides. The specific process for using chlorine in pesticide production can vary depending on the compound being produced.
- Refrigeration: Chlorine is used in the production of refrigerants, such as chlorofluorocarbons (CFCs), which were widely used in industrial refrigeration systems before they were phased out due to their impact on the ozone layer.
- Flame retardants: Chlorine is used in the production of flame retardants for textiles and plastics. The specific process for using chlorine in flame retardant production can vary depending on the material being treated.
Overall, the methods for using chlorine can vary widely depending on the specific application, but they often involve using chlorine gas or chlorine compounds in a reaction or treatment process.
Nomenclature of p-Block Elements Group 17 Uses of chlorine
The elements in Group 17 of the periodic table, including chlorine, follow a systematic nomenclature system called the IUPAC (International Union of Pure and Applied Chemistry) naming system. The naming system follows the following rules:
- The name of the element is simply the name of the element itself.
- When referring to an ion of the element, the suffix “-ide” is added to the stem of the element name. For example, chloride is the anion of chlorine.
- For cations formed from Group 17 elements, the word “ion” is added to the stem of the element name. For example, a cation of chlorine would be called a chloronium ion.
- When referring to compounds containing Group 17 elements, the name of the element with the “-ide” suffix is used, followed by the name of the other element or group. For example, sodium chloride is the compound formed from sodium and chlorine.
- If the Group 17 element forms more than one anion or oxidation state, the charge of the ion is indicated using Roman numerals in parentheses after the element name. For example, FeCl3 is iron(III) chloride, indicating that the iron ion has a +3 charge.
In summary, the nomenclature of Group 17 elements, including chlorine, follows a systematic naming system based on the IUPAC rules, which involves using the element name, adding the “-ide” suffix for anions, and indicating the charge of the ion using Roman numerals when necessary.
Structures of p-Block Elements Group 17 Uses of chlorine
The elements in Group 17 of the periodic table, including chlorine, have unique structures and bonding patterns due to their electron configurations. Chlorine has an atomic number of 17 and a valence electron configuration of [Ne]3s2 3p5, which means it has 7 valence electrons in the outermost p orbital. These electrons are involved in the formation of chemical bonds with other atoms or molecules.
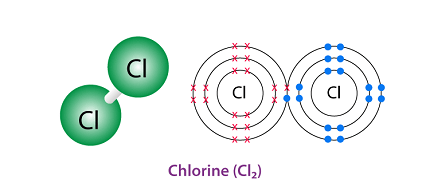
Chlorine exists as a diatomic molecule in its elemental form, with two chlorine atoms sharing a covalent bond. The structure of chlorine molecule can be represented as Cl-Cl, where each chlorine atom contributes one electron to the bond, forming a single covalent bond.
In addition to its diatomic molecule form, chlorine can also form compounds with other elements. One example is sodium chloride (NaCl), which is a common compound formed from the reaction between chlorine and sodium. Sodium chloride has a crystalline structure, with each sodium ion surrounded by six chloride ions and each chloride ion surrounded by six sodium ions, forming a lattice structure.
Another example is hydrogen chloride (HCl), which is a gas at room temperature and forms a polar covalent bond with hydrogen. The molecule has a linear shape, with the hydrogen and chlorine atoms arranged in a straight line.
In summary, the structures of Group 17 elements and their compounds, including chlorine, depend on the electron configurations and bonding patterns of the atoms involved. Chlorine exists as a diatomic molecule with a single covalent bond, and can form compounds with other elements, such as sodium and hydrogen, with unique crystal or molecular structures.
Case Study on p-Block Elements Group 17 Uses of chlorine
One interesting case study on the uses of Group 17 element chlorine is its role in water treatment. Chlorine is commonly used as a disinfectant in water treatment plants, where it is added to water to kill harmful bacteria, viruses, and other microorganisms that can cause illness or disease. The use of chlorine in water treatment has been a major public health advancement, helping to prevent the spread of waterborne illnesses and diseases.
Chlorine works by disrupting the cellular structure and metabolic processes of microorganisms, ultimately leading to their death. It does this by reacting with organic matter and proteins in the cell walls of microorganisms, causing them to break down and lose their ability to function. Chlorine is also effective against viruses, which are not technically alive but can still be deactivated by the chemical reactions caused by chlorine.
The use of chlorine in water treatment has been met with some controversy due to concerns about the potential health risks of exposure to the chemical. Chlorine can react with other substances in the water to form byproducts, such as trihalomethanes (THMs), which have been linked to an increased risk of cancer and other health problems. However, studies have shown that the benefits of using chlorine to disinfect water far outweigh the risks of exposure to THMs and other byproducts.
To mitigate the potential health risks associated with chlorine, water treatment plants carefully monitor the levels of chlorine in the water and take steps to minimize the formation of THMs and other byproducts. In addition, alternative water treatment methods, such as ozonation or ultraviolet (UV) light treatment, can be used in combination with or as a replacement for chlorine disinfection.
In summary, the use of chlorine as a disinfectant in water treatment is an important case study on the uses of Group 17 element chlorine. While concerns about potential health risks associated with exposure to chlorine byproducts exist, the benefits of using chlorine to prevent waterborne illnesses and diseases are significant and continue to be recognized as an important public health advancement.
White paper on p-Block Elements Group 17 Uses of chlorine
Introduction:
The p-block elements in the periodic table include a group of elements known as Group 17 or the halogens. The most common and abundant halogen is chlorine, which is widely used in various industrial and consumer applications due to its unique properties. This white paper will explore the various uses of chlorine and its compounds in different industries and applications.
Water Treatment:
One of the most important uses of chlorine is in water treatment, where it is used to disinfect water and kill harmful microorganisms, including bacteria and viruses. Chlorine is added to the water supply in small quantities to kill bacteria and other microorganisms that can cause diseases such as cholera, typhoid fever, and dysentery. Chlorine works by breaking down the cellular structure of these microorganisms and disrupting their metabolic processes, ultimately leading to their death. While concerns about the potential health risks of exposure to chlorine byproducts such as trihalomethanes (THMs) exist, the benefits of using chlorine in water treatment far outweigh the risks.
Pulp and Paper Manufacturing:
Chlorine is also used in the pulp and paper manufacturing industry as a bleaching agent to whiten wood pulp and paper. The process involves adding chlorine gas to the pulp, which reacts with lignin, a component of wood that gives it its brown color, to break it down and remove it. Chlorine gas is a highly reactive oxidizing agent that can bleach wood pulp and paper effectively. However, the use of chlorine in this industry has declined due to concerns about the environmental impact of chlorine and its compounds.
Pharmaceuticals:
Chlorine is also used in the pharmaceutical industry to produce medicines and other drugs. Chlorine is a key ingredient in the production of various drugs, including antibiotics, analgesics, and antiseptics. Chlorine can be used to modify the chemical structure of drugs and make them more effective. Chlorine is also used to sterilize equipment and clean surfaces in pharmaceutical manufacturing facilities.
Organic Chemistry:
Chlorine is a highly reactive element that can form compounds with a wide range of organic molecules, making it useful in organic chemistry. Chlorine can be used to substitute hydrogen atoms in organic molecules, leading to the formation of new compounds with different properties. Chlorine is also used in the synthesis of other chemicals, including dyes, solvents, and plastics.
Conclusion:
In conclusion, chlorine is a versatile element with many important uses in various industries and applications. The most significant use of chlorine is in water treatment, where it is used to disinfect water and prevent the spread of waterborne diseases. Chlorine is also used in the pulp and paper manufacturing industry, the pharmaceutical industry, and organic chemistry. While concerns about the potential health and environmental risks of chlorine and its compounds exist, the benefits of using chlorine in various industries and applications far outweigh the risks.