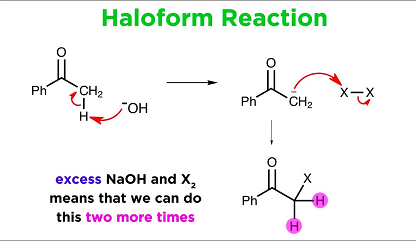
The haloform reaction is a chemical reaction in which a methyl ketone (a compound containing a carbonyl group, C=O, bonded to a methyl group, CH3) is treated with a halogen (chlorine, bromine, or iodine) and a strong base (usually sodium hydroxide, NaOH) to produce a carboxylic acid and a haloform (a compound containing a halogen atom bonded to a carbonyl group, CX3-C=O).
The general equation for the haloform reaction is:
R-COCH3 + 3X2 + 4OH- → R-CO2- + X3-COH + 2H2O + 2X-
where R represents a hydrocarbon chain (e.g. methyl, ethyl, propyl, etc.), X represents a halogen atom (Cl, Br, or I), and OH- represents a hydroxide ion.
The haloform reaction is useful in the laboratory for the detection of methyl ketones. When a methyl ketone is treated with halogen and base, the haloform is produced as a byproduct, which can be easily detected by its characteristic odor and yellow color. This reaction is also used in the industrial production of certain organic compounds, such as iodoform (CHI3) and bromoform (CHBr3).
What is Required Aldehydes and Ketones Haloform reaction
The haloform reaction requires aldehydes and methyl ketones (also called 2-ketones) in order to occur.
In the case of aldehydes, they can undergo the haloform reaction only when they have at least one methyl group attached to the carbonyl carbon. This is because the reaction requires a methyl group to be oxidized to a carboxylate ion, which then reacts with the halogen to form the haloform.
In the case of ketones, only methyl ketones can undergo the haloform reaction, since they have a methyl group attached to one of the carbonyl carbons. Ketones lacking a methyl group, such as ethyl ketone or propyl ketone, will not undergo the haloform reaction.
It’s worth noting that not all aldehydes and methyl ketones will undergo the haloform reaction under standard conditions. The reaction is most effective with methyl ketones that have electron-withdrawing substituents, such as halogens or nitro groups, on the aromatic ring. In general, aldehydes and methyl ketones with more reactive carbonyl groups will undergo the reaction more readily.
When is Required Aldehydes and Ketones Haloform reaction
The haloform reaction is used for several purposes in both laboratory and industrial settings. Here are some examples of when the haloform reaction might be used:
- To detect the presence of a methyl ketone or an aldehyde with a methyl group: The haloform reaction is commonly used in the laboratory to detect the presence of a methyl ketone or an aldehyde with a methyl group. When a sample suspected of containing a methyl ketone or aldehyde is treated with iodine and a strong base such as sodium hydroxide, the haloform reaction occurs only if the compound contains a methyl group attached to the carbonyl carbon. The formation of a yellow precipitate, which is the haloform compound, indicates the presence of the methyl group.
- To synthesize haloforms: The haloform reaction is also used in the industrial production of haloforms such as chloroform, bromoform, and iodoform. In this case, a methyl ketone or aldehyde is treated with a halogen and a strong base to form the corresponding haloform.
- To oxidize a methyl group to a carboxylate ion: In certain organic synthesis reactions, it may be necessary to convert a methyl group to a carboxylate ion. The haloform reaction can be used to achieve this transformation by treating the compound with iodine and a strong base.
Overall, the haloform reaction is a useful tool in organic chemistry for identifying, synthesizing, and transforming compounds containing a methyl group attached to a carbonyl carbon.
Where is Required Aldehydes and Ketones Haloform reaction
The haloform reaction can be carried out in a variety of laboratory and industrial settings. In the laboratory, the reaction is typically carried out in a small-scale reaction vessel such as a test tube or round-bottom flask. The reaction mixture typically consists of the aldehyde or ketone being tested, a halogen source such as iodine or bromine, and a strong base such as sodium hydroxide. The reaction can be monitored by observing the formation of a yellow precipitate, which is the haloform product.
In industrial settings, the haloform reaction is often carried out in larger-scale reactors under controlled conditions. For example, in the production of chloroform, a methyl ketone is treated with chlorine gas and sodium hydroxide in a reactor vessel. The reaction is carried out under carefully controlled conditions of temperature, pressure, and reactant concentrations to optimize the yield of the desired haloform product.
Overall, the haloform reaction can be carried out in a variety of laboratory and industrial settings depending on the desired application.
How is Required Aldehydes and Ketones Haloform reaction
The haloform reaction is typically carried out by mixing an aldehyde or a methyl ketone with a halogen source and a strong base in a reaction vessel. Here are the general steps involved in carrying out the reaction:
- Preparation of the reaction mixture: The aldehyde or methyl ketone is dissolved in a suitable solvent, such as ethanol or acetone. A halogen source such as iodine or bromine is added to the solution, followed by a strong base such as sodium hydroxide.
- Reaction: The mixture is stirred or shaken to ensure thorough mixing of the reagents. The reaction typically proceeds rapidly, and a yellow precipitate of the haloform compound forms within a few minutes.
- Workup: The reaction mixture is typically acidified to neutralize the excess base and quench the reaction. The haloform product is then extracted into an organic solvent such as ether or chloroform.
- Purification: The organic extract is dried over anhydrous sodium sulfate and filtered to remove any solids. The solvent is then evaporated under reduced pressure to yield the pure haloform product.
It’s worth noting that the reaction conditions, such as the choice of solvent, halogen source, and base, can be varied depending on the specific application. Additionally, care should be taken when handling the halogen and strong base, as they can be corrosive and harmful if not handled properly.
Production of Aldehydes and Ketones Haloform reaction
The haloform reaction is not typically used for the production of aldehydes and ketones, as the reaction typically results in the degradation of these compounds rather than their synthesis. However, the haloform reaction can be used to synthesize haloforms such as chloroform, bromoform, and iodoform, which are commonly used as solvents, reagents, and pharmaceutical intermediates.
The production of haloforms typically involves the reaction of a methyl ketone or aldehyde with a halogen and a strong base. For example, chloroform can be produced by reacting acetone with chlorine gas and sodium hydroxide in a reaction vessel. The reaction is typically carried out under carefully controlled conditions of temperature, pressure, and reactant concentrations to optimize the yield of the desired haloform product.
Overall, while the haloform reaction is not typically used for the production of aldehydes and ketones, it can be a useful method for the synthesis of haloforms for various industrial applications.
Case Study on Aldehydes and Ketones Haloform reaction
One application of the haloform reaction is in the detection of acetone in biological samples. Acetone is a common metabolite in the human body, and elevated levels of acetone in the blood or urine can be a sign of certain medical conditions such as diabetes or ketoacidosis. The haloform reaction can be used to detect the presence of acetone in these samples.
In this case study, let’s consider the use of the haloform reaction in the detection of acetone in urine samples. The general steps involved in this application are as follows:
- Collection of urine samples: Urine samples are collected from patients suspected of having elevated levels of acetone in their urine.
- Preparation of the reaction mixture: To a small amount of the urine sample, a halogen source such as iodine and a strong base such as sodium hydroxide are added. The mixture is then shaken to ensure thorough mixing of the reagents.
- Reaction: The reaction typically proceeds rapidly, and a yellow precipitate of iodoform, which is the haloform compound, forms within a few minutes if acetone is present in the sample.
- Observation of results: The presence of a yellow precipitate indicates the presence of acetone in the sample. The intensity of the color can be used to estimate the concentration of acetone in the sample.
- Confirmation of results: To confirm the presence of acetone, other tests such as gas chromatography-mass spectrometry (GC-MS) can be performed.
Overall, the haloform reaction is a useful tool in the detection of acetone in biological samples such as urine. It is a simple, rapid, and cost-effective method that can provide valuable information in the diagnosis and treatment of medical conditions such as diabetes and ketoacidosis.
White paper on Aldehydes and Ketones Haloform reaction
Introduction:
The haloform reaction is a well-known organic reaction that involves the conversion of an aldehyde or a methyl ketone into a haloform compound, typically chloroform, bromoform, or iodoform. This reaction is commonly used in the laboratory for the synthesis of haloforms, as well as for the detection of acetone in biological samples. In this white paper, we will discuss the mechanism, applications, and limitations of the haloform reaction.
Mechanism:
The haloform reaction typically proceeds through a series of steps that involve the following stages:
- Halogenation: The aldehyde or methyl ketone is halogenated by a halogen source such as iodine, bromine, or chlorine.
- Enolization: The halogenated compound is converted into its enol form by the addition of a strong base such as sodium hydroxide.
- Haloform formation: The enol undergoes a halogen exchange reaction with the halogen source to yield the haloform compound, which precipitates out of the solution as a yellow or white solid.
Applications:
The haloform reaction has a wide range of applications in both industry and academia. Some of the major applications of this reaction include:
- Synthesis of haloforms: The haloform reaction is a useful method for the synthesis of chloroform, bromoform, and iodoform, which are important industrial solvents, reagents, and pharmaceutical intermediates.
- Detection of acetone: The haloform reaction is commonly used in the detection of acetone in biological samples such as urine. Elevated levels of acetone in the blood or urine can be a sign of certain medical conditions such as diabetes or ketoacidosis.
- Synthesis of carboxylic acids: The haloform reaction can also be used to synthesize carboxylic acids from aldehydes or methyl ketones.
- Stereospecific synthesis: The haloform reaction can be used for the stereospecific synthesis of chiral molecules.
Limitations:
While the haloform reaction has many useful applications, it also has several limitations that should be taken into account. Some of the major limitations of this reaction include:
- Degradation of the starting material: The haloform reaction typically results in the degradation of the starting material rather than its synthesis. This limits the applicability of the reaction to certain types of compounds.
- Waste generation: The haloform reaction generates a large amount of waste, particularly when it is used for the synthesis of haloforms. This can be a significant environmental concern.
- Safety considerations: The haloform reaction involves the use of strong bases and halogen sources, which can be corrosive and harmful if not handled properly. Care should be taken to ensure safe handling and disposal of these reagents.
Conclusion:
The haloform reaction is a versatile and widely used organic reaction that has a wide range of applications in both industry and academia. While it has some limitations, it remains a valuable tool for the synthesis of haloforms, the detection of acetone in biological samples, and the stereospecific synthesis of chiral molecules. By understanding the mechanism and limitations of the haloform reaction, researchers can make informed decisions about when and how to use this important reaction.
