Halogenation of benzene refers to the substitution of one or more hydrogen atoms in a benzene ring with halogen atoms such as chlorine or bromine. The reaction is typically carried out in the presence of a halogen carrier such as iron or aluminum chloride, which helps activate the halogen and facilitate the substitution reaction. The reaction can be written as follows:
C6H6 + X2 -> C6H5X + HX
where X represents a halogen atom (Cl or Br), C6H5X is a halobenzene, and HX is hydrogen halide (HCl or HBr).
The reaction can be further optimized by using a mixture of the halogen and carrier in a solvent such as carbon tetrachloride or chloroform. The reaction typically proceeds with high selectivity for mono-substitution, meaning that only one hydrogen atom in the benzene ring is replaced with a halogen atom. Multiple substitutions can also occur under certain conditions, but these are typically less common and less desirable in most applications.
Halogenation of benzene is an important reaction in organic chemistry, as it is a key step in the synthesis of many important chemicals, including pharmaceuticals, agrochemicals, and plastics. It is also a fundamental reaction in the study of aromatic compounds and their reactivity.
What is Required Benzene Halogenation
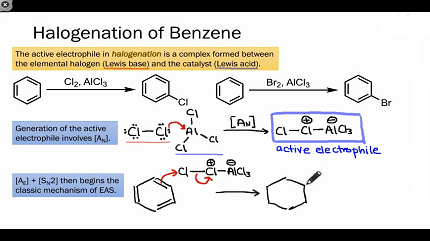
The halogenation of benzene requires a halogenating agent or halogen carrier, such as iron or aluminum halides, to facilitate the substitution reaction. The halogenating agent acts as a Lewis acid catalyst, which means it accepts electron pairs from the halogen and polarizes the carbon-halogen bond, making it more reactive towards the benzene ring.
The most commonly used halogenating agents for benzene halogenation are iron(III) chloride (FeCl3) and aluminum chloride (AlCl3). Iron(III) chloride is typically used for chlorination reactions, while aluminum chloride is used for bromination reactions. The choice of halogenating agent depends on the desired halogen and reaction conditions.
In addition to the halogenating agent, the reaction requires a halogen source such as chlorine gas (Cl2) or bromine (Br2). The halogen is typically added slowly to the reaction mixture to prevent overhalogenation and the formation of unwanted products.
Finally, a solvent is often used to dissolve the reactants and facilitate the reaction. Common solvents include chlorinated hydrocarbons such as carbon tetrachloride (CCl4) or chloroform (CHCl3), which can help stabilize the intermediate species formed during the reaction.
When is Required Benzene Halogenation
Benzene halogenation is used in organic synthesis to introduce halogen atoms (chlorine or bromine) onto a benzene ring. This reaction is used in the production of a wide range of halogenated organic compounds, including pharmaceuticals, agrochemicals, and specialty chemicals.
For example, chlorobenzene is an important intermediate in the production of many herbicides, insecticides, and fungicides. Bromobenzene is used as a solvent, as well as in the synthesis of pharmaceuticals, dyes, and fragrances.
Benzene halogenation is also used in the laboratory to study the reactivity of aromatic compounds and to synthesize new compounds. The reaction is often used as a model reaction for studying the mechanisms of electrophilic aromatic substitution reactions.
Overall, benzene halogenation is a versatile and widely used reaction in organic synthesis and is an important tool for the production of halogenated organic compounds.
Where is Required Benzene Halogenation
Benzene halogenation is a common reaction in organic synthesis and can be carried out in a laboratory setting or in an industrial-scale chemical production facility. The reaction can be performed using a variety of equipment, from simple round-bottom flasks to more complex reaction vessels and reactors.
In the laboratory, benzene halogenation is typically carried out using a reaction flask equipped with a reflux condenser and a magnetic stirrer. The reactants are added to the flask and heated to a specific temperature while being stirred. The reaction progress is monitored by various analytical techniques such as thin-layer chromatography, gas chromatography, or nuclear magnetic resonance spectroscopy.
In an industrial setting, benzene halogenation is often carried out in a continuous-flow reactor or a batch reactor. The reaction conditions, such as temperature, pressure, and reaction time, are carefully controlled to optimize the yield and selectivity of the desired product. The reaction is typically monitored in real-time using process analytical technology (PAT) to ensure consistent quality and productivity.
Benzene halogenation is used in a wide range of industries, including pharmaceuticals, agrochemicals, plastics, and specialty chemicals. It is an important reaction for the production of many everyday products, such as medicines, pesticides, and solvents.
How is Required Benzene Halogenation
The mechanism of benzene halogenation involves electrophilic aromatic substitution, where a halogen replaces a hydrogen atom on the benzene ring. The reaction is typically carried out in the presence of a halogenating agent, which acts as a Lewis acid catalyst to activate the halogen and facilitate the substitution reaction.
The general mechanism for benzene halogenation is as follows:
- Activation of the halogenating agent: The halogenating agent, such as iron(III) chloride or aluminum chloride, reacts with the halogen (chlorine or bromine) to form a complex that is more reactive than the halogen alone.
- Formation of the electrophile: The complex reacts with a molecule of the halogen to form an electrophilic halogen species, such as FeCl4+ or AlCl4-, which is highly reactive towards the benzene ring.
- Attack of the electrophile on the benzene ring: The electrophilic halogen species attacks the benzene ring, forming a highly unstable intermediate species known as a sigma complex.
- Rearrangement of the sigma complex: The sigma complex rearranges to form a more stable carbocation intermediate.
- Deprotonation of the carbocation intermediate: The carbocation intermediate is deprotonated by a chloride or bromide ion, forming the final product, a halogenated benzene.
The overall reaction can be represented as:
C6H6 + X2 + halogenating agent -> C6H5X + HX + halogenating agent
where X represents a halogen atom (Cl or Br), C6H5X is a halobenzene, and HX is hydrogen halide (HCl or HBr).
Nomenclature of Benzene Halogenation
The nomenclature of benzene halogenation involves naming the halogenated product based on the position of the halogen substituent(s) on the benzene ring. The halogen is indicated by a prefix (chloro- or bromo-) followed by a number indicating the position of the halogen on the ring relative to a chosen reference point.
For example, chlorobenzene has a single chlorine atom attached to the benzene ring, while 1,2-dichlorobenzene has two chlorine atoms attached to adjacent carbon atoms on the benzene ring. If there are more than two halogen atoms on the ring, the positions of all the halogens are indicated by a series of numbers separated by commas.
The numbering of the benzene ring follows the rules of the IUPAC (International Union of Pure and Applied Chemistry) system, where the carbon atoms are numbered sequentially, starting with the carbon atom closest to a substituent group, such as the halogen. If there are multiple substituents on the ring, the substituents are numbered in alphabetical order.
In addition to the prefix and number indicating the position of the halogen, the name of the parent hydrocarbon (benzene) is also included in the name of the halogenated product. For example, 1-bromo-4-chlorobenzene indicates that there is a bromine atom at position 1 and a chlorine atom at position 4 on the benzene ring.
Case Study on Benzene Halogenation
One example of the application of benzene halogenation is in the synthesis of pharmaceutical compounds. A case study of benzene halogenation in the synthesis of the antidepressant drug fluoxetine (Prozac) is provided below.
Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) that is used to treat depression, obsessive-compulsive disorder, and other mental health conditions. The synthesis of fluoxetine involves several steps, one of which is the halogenation of benzene.
The halogenation step involves the reaction of benzene with bromine in the presence of a Lewis acid catalyst, iron(III) bromide, to produce bromobenzene. The reaction is typically carried out under reflux conditions in a reaction flask equipped with a reflux condenser and a magnetic stirrer.
Once the bromobenzene is obtained, it undergoes several more steps to form the final product, fluoxetine. The synthesis of fluoxetine is a multistep process that involves the coupling of a phenylalanine derivative with a naphthalene ring system, followed by several steps of functionalization and reduction.
The halogenation step is important because it introduces a halogen substituent onto the benzene ring, which is a key intermediate in the subsequent steps of the synthesis. The halogen substituent is also important for the pharmacological properties of the final drug product, as it affects the binding of the drug to its target receptor.
In addition to its use in the synthesis of fluoxetine, benzene halogenation is also used in the synthesis of other pharmaceutical compounds, as well as in the production of agrochemicals, dyes, and other specialty chemicals. The reaction is an important tool for organic chemists and is widely used in both laboratory and industrial settings.
White paper on Benzene Halogenation
Introduction:
Benzene halogenation is a fundamental reaction in organic chemistry that involves the substitution of a hydrogen atom on a benzene ring with a halogen atom. The reaction is an electrophilic aromatic substitution that is typically carried out in the presence of a halogenating agent, such as iron(III) chloride or aluminum chloride. The resulting halogenated benzene compounds are important intermediates in the synthesis of many pharmaceuticals, agrochemicals, and specialty chemicals.
Applications:
Benzene halogenation has numerous applications in the synthesis of pharmaceutical compounds. For example, the antidepressant drug fluoxetine is synthesized via a multistep process that involves the halogenation of benzene to produce bromobenzene, which is then used as an intermediate in subsequent reactions.
In addition to pharmaceuticals, benzene halogenation is also used in the production of agrochemicals, such as herbicides and insecticides, as well as dyes and other specialty chemicals. The halogenated benzene compounds produced by the reaction can be further functionalized to introduce other chemical groups, such as amino or nitro groups, which can impart specific properties to the resulting compound.
Mechanism:
The mechanism of benzene halogenation involves the formation of an electrophilic halogen species that attacks the benzene ring, forming a highly unstable intermediate species known as a sigma complex. The sigma complex rearranges to form a more stable carbocation intermediate, which is then deprotonated by a halide ion to form the final halogenated benzene product.
The reaction is typically carried out in the presence of a halogenating agent, which acts as a Lewis acid catalyst to activate the halogen and facilitate the substitution reaction. The choice of halogenating agent can affect the reactivity and selectivity of the reaction, and different agents may be chosen depending on the specific requirements of the reaction.
Conclusion:
Benzene halogenation is a versatile reaction that is widely used in organic synthesis. The resulting halogenated benzene compounds are important intermediates in the production of many pharmaceuticals, agrochemicals, and specialty chemicals. The reaction mechanism involves electrophilic aromatic substitution, and the choice of halogenating agent can affect the reactivity and selectivity of the reaction. Overall, benzene halogenation is an important tool for organic chemists and plays a crucial role in the development of new chemical compounds.
