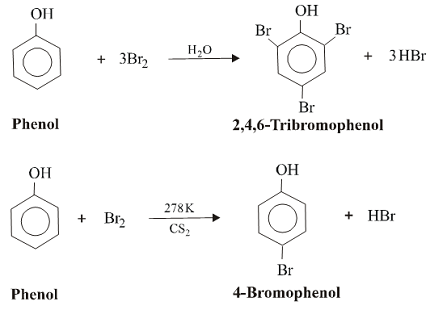
Halogenation of phenols involves the substitution of one or more hydrogen atoms on the phenolic ring with a halogen atom such as chlorine, bromine or iodine. The reaction is typically carried out in the presence of a Lewis acid catalyst such as iron(III) chloride or aluminum trichloride to increase the electrophilicity of the halogen.
The halogenation reaction can occur at either the ortho, para, or meta positions of the phenol ring, depending on the nature of the phenol and the halogen used. For example, when bromine is used as the halogenating agent, the reaction typically proceeds at the ortho and para positions, while chlorine tends to react primarily at the ortho position.
The general reaction mechanism for halogenation of phenols involves the formation of an electrophilic halogen species, which attacks the phenolic ring to form a halogenated intermediate. The intermediate then undergoes deprotonation to form the final halogenated product.
Halogenated phenols have a wide range of applications, including as intermediates in the synthesis of pharmaceuticals, dyes, and other organic compounds. However, some halogenated phenols are also known to be toxic and potentially carcinogenic, and their use is regulated by various environmental and health agencies.
What is Required Phenols Halogenation
In order to carry out halogenation of phenols, you will need:
- Phenol: Phenol is the starting material for the reaction.
- Halogenating agent: The most commonly used halogenating agents for phenol halogenation are chlorine, bromine, and iodine. These reagents are usually in the form of their respective halogens, such as chlorine gas or bromine liquid.
- Catalyst: A Lewis acid catalyst such as iron(III) chloride or aluminum trichloride is typically used to increase the electrophilicity of the halogen and facilitate the reaction.
- Solvent: A solvent is usually needed to dissolve the phenol and the halogenating agent. Common solvents include dichloromethane, chloroform, and carbon tetrachloride.
- Temperature and time: The reaction is typically carried out at room temperature or slightly above, and the reaction time can range from a few minutes to several hours, depending on the specific conditions.
Overall, phenol halogenation is a relatively simple reaction that can be carried out in a standard laboratory setting with commonly available reagents and equipment. However, care should be taken when handling halogenating agents, as they can be corrosive and potentially hazardous.
When is Required Phenols Halogenation
Phenol halogenation is used in organic synthesis to introduce halogen atoms into the phenol molecule, which can modify the properties of the compound and make it more useful for various applications. Some common reasons for performing phenol halogenation include:
- Synthesis of pharmaceuticals: Halogenated phenols are used as intermediates in the synthesis of a wide range of pharmaceuticals, including antibiotics, antiseptics, and anti-inflammatory drugs.
- Production of dyes and pigments: Halogenated phenols can be used to produce a variety of dyes and pigments for use in textiles, paints, and other industrial applications.
- Polymer production: Halogenated phenols can be used as monomers in the production of certain types of polymers, such as polycarbonates and polyesters.
- Environmental applications: Halogenated phenols are sometimes used as disinfectants and preservatives in water treatment and other environmental applications.
Overall, phenol halogenation is a versatile and widely used reaction that has many practical applications in organic synthesis and industrial chemistry.
Where is Required Phenols Halogenation
Phenol halogenation is a common organic reaction that can be carried out in a laboratory setting or in an industrial setting.
In a laboratory setting, phenol halogenation is typically performed using small quantities of reagents and equipment. The reaction can be carried out in a standard fume hood using commonly available laboratory glassware and solvents.
In an industrial setting, phenol halogenation may be carried out on a much larger scale using specialized equipment and facilities. For example, phenol halogenation may be performed in a chemical plant or refinery to produce halogenated phenols for use in the production of various chemicals, plastics, and other materials.
Overall, phenol halogenation is a versatile and widely used reaction that can be carried out in a variety of settings depending on the specific application and scale of the reaction.
How is Required Phenols Halogenation
Phenol halogenation can be carried out using a variety of different methods, depending on the specific application and desired product. However, a common method for phenol halogenation involves the use of a halogenating agent and a Lewis acid catalyst, such as iron(III) chloride or aluminum trichloride. The general reaction mechanism for phenol halogenation is as follows:
- Formation of the electrophilic halogen species: The halogenating agent (e.g. chlorine or bromine) reacts with the Lewis acid catalyst to form an electrophilic halogen species, such as FeCl4- or AlCl4-, which is capable of attacking the phenol ring.
- Attack on the phenol ring: The electrophilic halogen species attacks the phenol ring, which results in the formation of a halogenated intermediate.
- Deprotonation: The intermediate undergoes deprotonation, which results in the formation of the final halogenated product.
The position of the halogenation on the phenol ring can be controlled by adjusting the reaction conditions, such as the type of halogenating agent used, the catalyst, the solvent, and the temperature. For example, bromine tends to react primarily at the ortho and para positions, while chlorine tends to react primarily at the ortho position.
Overall, phenol halogenation is a relatively straightforward reaction that can be carried out using commonly available reagents and equipment in a laboratory or industrial setting. However, care should be taken when handling halogenating agents, as they can be corrosive and potentially hazardous.
Production of Phenols Halogenation
The production of halogenated phenols can be carried out using various methods, depending on the specific application and desired product. One common method for the production of halogenated phenols is the halogenation of phenol using a halogenating agent and a Lewis acid catalyst, as described in the previous answer.
In an industrial setting, the production of halogenated phenols typically involves large-scale batch or continuous processes using specialized equipment and facilities. For example, the production of chlorinated phenols may be carried out using a reactor vessel equipped with agitation, temperature control, and an inlet for the halogenating agent. The phenol and catalyst are added to the reactor, followed by the addition of the halogenating agent under controlled conditions. The reaction is typically monitored and controlled to optimize the yield and selectivity of the desired product.
The production of halogenated phenols may also involve post-treatment steps, such as washing, distillation, or purification, to remove impurities and isolate the desired product. The resulting halogenated phenol products can be used in a wide range of applications, including pharmaceuticals, dyes and pigments, and polymer production.
Overall, the production of halogenated phenols is a complex and specialized process that requires careful planning, optimization, and control to ensure consistent and high-quality products.
Case Study on Phenols Halogenation
One example of the use of phenol halogenation is in the production of the disinfectant and antiseptic compound chloroxylenol (PCMX). Chloroxylenol is commonly used in household cleaning products and in healthcare settings to kill bacteria and other microorganisms.
The production of chloroxylenol involves the halogenation of 4-chlorophenol, which is carried out using a chlorinating agent and a Lewis acid catalyst. The reaction can be carried out in a batch or continuous process using specialized equipment and facilities.
In one typical process, 4-chlorophenol is mixed with a chlorinating agent, such as chlorine gas or sodium hypochlorite, and a Lewis acid catalyst, such as aluminum trichloride. The reaction mixture is heated and agitated under controlled conditions to optimize the yield and selectivity of the desired product.
After the reaction is complete, the resulting mixture is washed, distilled, and purified to isolate the chloroxylenol product. The final product is typically a white or off-white crystalline powder that can be further processed and formulated into various products, such as liquid disinfectants, soaps, and lotions.
Overall, the halogenation of phenols is a versatile and widely used reaction that has many practical applications in organic synthesis and industrial chemistry. The production of chloroxylenol is just one example of the many useful compounds that can be synthesized using phenol halogenation.
White paper on Halogenation
Introduction:
Halogenation is a chemical reaction that involves the addition of a halogen atom to a molecule. The most common halogens used in halogenation reactions are fluorine, chlorine, bromine, and iodine. Halogenation reactions are widely used in organic chemistry and have a variety of important applications in industry and in everyday life.
Halogenation Reactions:
Halogenation reactions can be classified into two main types: radical halogenation and electrophilic halogenation.
- Radical halogenation: In radical halogenation reactions, a halogen atom reacts with a molecule in the presence of light or heat to produce a free radical. The free radical then reacts with another molecule to form a halogenated product. Examples of radical halogenation reactions include the chlorination of methane to produce chloromethane and the bromination of propane to produce 1-bromopropane.
- Electrophilic halogenation: In electrophilic halogenation reactions, a halogenating agent reacts with a molecule in the presence of a Lewis acid catalyst to produce an electrophilic halogen species. The electrophilic halogen species then attacks the molecule to form a halogenated product. Examples of electrophilic halogenation reactions include the bromination of benzene to produce bromobenzene and the chlorination of acetic acid to produce chloroacetic acid.
Applications of Halogenation:
Halogenation reactions have many important applications in industry and in everyday life. Some examples include:
- Production of pharmaceuticals: Many pharmaceuticals contain halogen atoms, which can enhance their biological activity and increase their stability. Halogenation reactions are often used in the synthesis of pharmaceuticals to introduce halogen atoms into the molecule.
- Production of plastics: Halogenated compounds are often used as flame retardants in plastics. Halogenation reactions are used to introduce halogen atoms into the plastic molecule, which can improve its fire resistance.
- Production of disinfectants: Halogenated compounds such as chloroxylenol and chlorhexidine are used as disinfectants and antiseptics. These compounds are often produced through halogenation reactions.
- Organic synthesis: Halogenation reactions are commonly used in organic synthesis to introduce halogen atoms into a molecule. This can change the reactivity and properties of the molecule, allowing it to be used in a wide range of applications.
Conclusion:
Halogenation reactions are an important class of chemical reactions with many applications in industry and in everyday life. Radical halogenation and electrophilic halogenation are two common types of halogenation reactions. Halogenation reactions are used in the production of pharmaceuticals, plastics, disinfectants, and in organic synthesis.