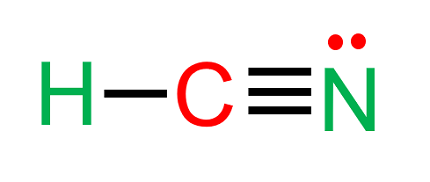
HCN stands for Hydrogen Cyanide, which is a colorless, highly poisonous gas that has a faint, bitter almond odor. It is used in a variety of industrial processes, including the production of synthetic fibers, plastics, and dyes. HCN is also found in nature, being produced by certain plants and some bacteria.
HCN is a deadly poison that can interfere with the body’s ability to use oxygen, leading to rapid unconsciousness and death. It can be lethal in concentrations as low as 100 parts per million (ppm). HCN exposure can occur through inhalation, ingestion, or skin contact, and symptoms of poisoning include headache, dizziness, confusion, nausea, and vomiting.
HCN is considered a chemical warfare agent and has been used as a method of execution in some countries. It is also used in the production of certain pesticides and rodenticides, and as a fumigant for stored grains and other food products. Because of its high toxicity, HCN must be handled with extreme caution and appropriate safety measures should be taken to prevent exposure.
What is Required Aldehydes and Ketones HCN
Aldehydes and ketones can react with hydrogen cyanide (HCN) to form cyanohydrins. The reaction requires the presence of a basic catalyst, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), to deprotonate the HCN and make it more reactive towards the carbonyl group in the aldehyde or ketone.
The reaction between HCN and aldehydes or ketones is an important synthetic method for the preparation of cyanohydrins, which are versatile building blocks for the synthesis of a variety of organic compounds. Cyanohydrins can be hydrolyzed to form carboxylic acids or reduced to form primary amines. Additionally, cyanohydrins can be used as intermediates in the synthesis of α-hydroxy acids, amino acids, and other important organic compounds.
The reaction between HCN and aldehydes or ketones is typically carried out under mild conditions, with the reactants being mixed in a solvent such as ethanol or water at room temperature. The reaction can be monitored by TLC (thin-layer chromatography) or IR (infrared) spectroscopy to ensure complete conversion of the starting material to the desired product.
When is Required Aldehydes and Ketones HCN
The reaction between aldehydes or ketones and hydrogen cyanide (HCN) is typically carried out in organic synthesis when the desired product is a cyanohydrin. Cyanohydrins are important building blocks for the synthesis of a variety of organic compounds, including carboxylic acids, amino acids, and α-hydroxy acids.
Some specific examples of when this reaction may be used include:
- Synthesis of α-hydroxy acids: The reaction between HCN and aldehydes or ketones can be used to synthesize α-hydroxy acids, which are important building blocks in the synthesis of many natural products and pharmaceuticals. For example, the reaction between HCN and benzaldehyde can be used to prepare mandelic acid, which is used as a chiral resolving agent.
- Synthesis of amino acids: Cyanohydrins can be hydrolyzed to form amino acids, which are important building blocks for proteins and other biologically active molecules. For example, the reaction between HCN and propanal can be used to prepare alanine, which is one of the 20 amino acids commonly found in proteins.
- Synthesis of pharmaceuticals: The reaction between HCN and aldehydes or ketones can be used in the synthesis of a variety of pharmaceuticals, including antidiabetic drugs, antiviral drugs, and anticancer agents. For example, the reaction between HCN and acetone can be used to prepare the antiviral drug ribavirin.
It is worth noting that the use of HCN in organic synthesis requires careful handling due to its high toxicity. Appropriate safety measures should be taken to prevent exposure to HCN and other hazardous materials.
Where is Required Aldehydes and Ketones HCN
The reaction between aldehydes or ketones and hydrogen cyanide (HCN) can be carried out in a laboratory setting, typically in a fume hood due to the toxic nature of HCN. The reaction can be performed in a variety of solvents, including water, ethanol, or methanol.
In industry, the reaction may be carried out on a larger scale in specialized chemical plants under carefully controlled conditions. The reaction may be performed in a reactor vessel equipped with temperature and pressure controls, as well as safety features to prevent accidental release of HCN or other hazardous materials.
The use of HCN in organic synthesis is strictly regulated due to its high toxicity and potential for misuse. In many countries, special permits or licenses are required to handle and use HCN. Additionally, appropriate safety measures, such as protective equipment, should be used to prevent exposure to HCN and other hazardous materials.
How is Required Aldehydes and Ketones HCN
The reaction between aldehydes or ketones and hydrogen cyanide (HCN) to form cyanohydrins can be performed using a variety of methods. Here is a general overview of the steps involved in the reaction:
- Preparation of the reagents: The aldehyde or ketone is dissolved in a solvent, such as ethanol or water, along with a basic catalyst, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH). Hydrogen cyanide is usually prepared in situ by adding an acid, such as hydrochloric acid (HCl), to a cyanide salt, such as sodium cyanide (NaCN), in the presence of water.
- Mixing of the reagents: The hydrogen cyanide is slowly added to the solution of the aldehyde or ketone and catalyst, usually under mild conditions such as room temperature and atmospheric pressure. The reaction mixture may be stirred or heated to promote the reaction.
- Workup and purification: The reaction mixture is typically quenched with water or a weak acid, such as acetic acid, to destroy any remaining hydrogen cyanide. The desired product, the cyanohydrin, can be isolated by filtration, extraction, or distillation, depending on the nature of the reaction and the product.
Overall, the reaction between aldehydes or ketones and HCN to form cyanohydrins is a versatile and useful method in organic synthesis. However, the use of HCN requires careful handling and appropriate safety measures to prevent exposure to this highly toxic compound.
Nomenclature of Aldehydes and Ketones HCN
The nomenclature of aldehydes and ketones that undergo reaction with hydrogen cyanide (HCN) to form cyanohydrins follows the general rules of organic nomenclature. Here are some guidelines:
- Aldehydes: Aldehydes have a functional group consisting of a carbonyl group (-C=O) bonded to a hydrogen atom (-H). In IUPAC nomenclature, aldehydes are named by replacing the “-e” ending of the parent hydrocarbon with “-al”. For example, formaldehyde, the simplest aldehyde, is also known as methanal.
When an aldehyde undergoes reaction with HCN to form a cyanohydrin, the product is named as the N-substituted form of the parent aldehyde, with the suffix “-cyanohydrin”. For example, benzaldehyde can react with HCN to form benzyl cyanohydrin.
- Ketones: Ketones have a functional group consisting of a carbonyl group (-C=O) bonded to two carbon atoms. In IUPAC nomenclature, ketones are named by replacing the “-e” ending of the parent hydrocarbon with “-one”. For example, acetone, the simplest ketone, is also known as propanone.
When a ketone undergoes reaction with HCN to form a cyanohydrin, the product is named as the N-substituted form of the parent ketone, with the suffix “-cyanohydrin”. For example, acetone can react with HCN to form 2-hydroxy-2-methylbutanenitrile, also known as acetone cyanohydrin.
In summary, the nomenclature of aldehydes and ketones that undergo reaction with HCN to form cyanohydrins involves naming the parent aldehyde or ketone according to IUPAC rules, followed by the suffix “-cyanohydrin” to indicate the presence of the cyanohydrin functional group.
Case Study on Aldehydes and Ketones HCN
One application of aldehydes and ketones undergoing reaction with hydrogen cyanide (HCN) to form cyanohydrins is in the production of synthetic fibers, specifically nylon-6,6.
Nylon-6,6 is a type of polyamide that is widely used in textiles, carpets, and industrial applications due to its strength, durability, and resistance to abrasion and chemicals. The production of nylon-6,6 involves several steps, one of which is the synthesis of adiponitrile, a key intermediate, from adipic acid.
Adiponitrile can be synthesized from adipic acid by reacting it with HCN in the presence of a catalyst, typically sulfuric acid or a metal cyanide. The reaction between adipic acid and HCN forms adiponitrile cyanohydrin, which can be hydrolyzed to adiponitrile.
The overall reaction can be represented as follows:
Adipic acid + HCN → Adiponitrile cyanohydrin → Adiponitrile + H2O
The adiponitrile is then further processed to form hexamethylenediamine, which is combined with adipic acid to form nylon-6,6.
The reaction between adipic acid and HCN to form adiponitrile cyanohydrin is an example of the aldehydes and ketones HCN reaction, which is an important application of this reaction in industry. The use of HCN in this process requires careful handling and appropriate safety measures to prevent exposure to this highly toxic compound.
In summary, the aldehydes and ketones HCN reaction has important industrial applications, such as in the production of nylon-6,6, which is a widely used synthetic fiber.
White paper on Aldehydes and Ketones HCN
Introduction:
Aldehydes and ketones are organic compounds that contain a carbonyl group, which is a carbon atom double-bonded to an oxygen atom. These functional groups undergo a variety of reactions, including the reaction with hydrogen cyanide (HCN) to form cyanohydrins. The aldehydes and ketones HCN reaction is an important industrial process with numerous applications, such as in the production of synthetic fibers, pharmaceuticals, and agrochemicals.
Production of Cyanohydrins:
The aldehydes and ketones HCN reaction involves the addition of HCN to the carbonyl group of an aldehyde or ketone, followed by the addition of a hydroxyl group (-OH) to the resulting intermediate to form a cyanohydrin. The reaction is typically carried out in the presence of a catalyst, such as sulfuric acid or a metal cyanide.
Cyanohydrins are useful intermediates in the synthesis of a variety of compounds, such as amino acids, peptides, and pharmaceuticals. For example, the synthesis of the anti-cancer drug Taxol involves the use of a cyanohydrin intermediate.
Applications of Cyanohydrins:
One of the most important applications of the aldehydes and ketones HCN reaction is in the production of synthetic fibers, such as nylon-6,6. Adipic acid, a key intermediate in the production of nylon-6,6, can be synthesized from HCN and adipic acid through the formation of adiponitrile cyanohydrin, which is then hydrolyzed to form adiponitrile.
Cyanohydrins are also used in the production of agrochemicals, such as herbicides and insecticides. For example, the herbicide bromoxynil is synthesized from the cyanohydrin of 3,5-dibromo-4-hydroxybenzaldehyde.
In addition, cyanohydrins have been used in the synthesis of pharmaceuticals, such as anti-inflammatory drugs and analgesics. The use of cyanohydrins as intermediates in pharmaceutical synthesis allows for the production of complex molecules with high efficiency and yield.
Safety Considerations:
The use of hydrogen cyanide in the aldehydes and ketones HCN reaction requires appropriate safety measures to prevent exposure to this highly toxic compound. Hydrogen cyanide is a deadly poison that can cause rapid death due to respiratory failure. In addition, HCN is flammable and explosive under certain conditions.
Conclusion:
The aldehydes and ketones HCN reaction is an important industrial process with numerous applications in the production of synthetic fibers, agrochemicals, and pharmaceuticals. The use of cyanohydrins as intermediates in these processes allows for the production of complex molecules with high efficiency and yield. However, the use of hydrogen cyanide in this reaction requires appropriate safety measures to prevent exposure to this highly toxic compound.
