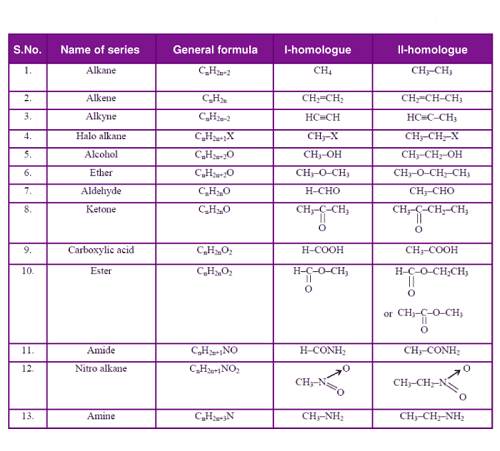
A homologous series is a group of organic compounds that have similar structures and chemical properties, with each successive member of the series differing from the previous one by a common structural unit, such as a -CH2- group. These compounds have the same functional group and similar chemical reactions, which result in similar physical and chemical properties.
The members of a homologous series have a similar molecular formula, and their physical and chemical properties vary in a regular and predictable manner with the increasing number of carbon atoms in the molecule. For example, the homologous series of alkanes includes compounds such as methane, ethane, propane, butane, pentane, and so on. Each successive member of the series differs from the previous one by a -CH2- unit, and the physical properties of the compounds, such as boiling point and melting point, increase as the number of carbon atoms in the molecule increases.
Homologous series are important in organic chemistry because they provide a systematic way of studying and predicting the properties and reactions of organic compounds. They also allow chemists to develop new compounds with desired properties by modifying the structure of existing compounds in the series.
What is Required Alkanes Homologous series
The Required Alkanes Homologous Series is a group of organic compounds that consists of only the alkane hydrocarbons. Alkanes are saturated hydrocarbons, which means they contain only single bonds between carbon atoms and hydrogen atoms. The molecular formula for alkanes is CnH2n+2, where n is the number of carbon atoms in the molecule.
The Required Alkanes Homologous Series includes compounds such as methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), hexane (C6H14), heptane (C7H16), octane (C8H18), nonane (C9H20), decane (C10H22), and so on. Each successive member of the series differs from the previous one by a -CH2- unit.
The physical properties of the Required Alkanes Homologous Series, such as boiling point and melting point, increase as the number of carbon atoms in the molecule increases. This is due to the increase in the molecular weight and size of the molecules, which leads to stronger intermolecular forces between the molecules. Alkanes are commonly used as fuels because they burn cleanly, producing carbon dioxide and water vapor as the only byproducts.
When is Required Alkanes Homologous series
The Required Alkanes Homologous Series is a specific type of homologous series that consists of a group of organic compounds called alkanes. Alkanes are a type of hydrocarbon that contains only carbon-carbon single bonds and are saturated with hydrogen atoms.
The Required Alkanes Homologous Series includes compounds such as methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), hexane (C6H14), heptane (C7H16), octane (C8H18), and so on. Each successive member of the series differs from the previous one by a -CH2- unit.
The Required Alkanes Homologous Series is important in organic chemistry because it provides a systematic way of studying and predicting the properties and reactions of alkanes. The physical properties of alkanes, such as melting point and boiling point, increase as the number of carbon atoms in the molecule increases. This is due to the increase in molecular weight and size of the molecules, which leads to stronger intermolecular forces between the molecules.
The Required Alkanes Homologous Series is also useful in the study of organic reactions, as the chemical reactivity of alkanes is primarily determined by the types of functional groups present in the molecule. Overall, the Required Alkanes Homologous Series is a fundamental concept in organic chemistry that allows chemists to understand and predict the behavior of alkanes and their derivatives.
Where is Required Alkanes Homologous series
The Required Alkanes Homologous Series is a concept in organic chemistry that describes a group of organic compounds called alkanes. Alkanes are a class of hydrocarbons that contain only carbon-carbon single bonds and are saturated with hydrogen atoms.
The Required Alkanes Homologous Series is found in various fields of study where organic chemistry plays a role, such as in the chemical industry, pharmaceuticals, and biochemistry. Alkanes are commonly used as fuels because they burn cleanly, producing carbon dioxide and water vapor as the only byproducts. The Required Alkanes Homologous Series also finds use in the production of polymers, waxes, and lubricants.
In addition to its practical applications, the Required Alkanes Homologous Series is an important concept in organic chemistry because it provides a systematic way of studying and predicting the properties and reactions of alkanes. The physical properties of alkanes, such as boiling point and melting point, increase as the number of carbon atoms in the molecule increases. This regular pattern of properties allows chemists to predict the behavior of alkanes and design new compounds with desired properties by modifying the structure of existing compounds in the series.
Overall, the Required Alkanes Homologous Series is a fundamental concept in organic chemistry that has broad applications in various fields of study and plays a critical role in understanding the properties and behavior of alkanes and their derivatives.
How is Required Alkanes Homologous series
The Required Alkanes Homologous Series is a group of organic compounds that share a common structure and chemical properties. Specifically, the Required Alkanes Homologous Series includes a group of hydrocarbons known as alkanes, which are composed of carbon and hydrogen atoms with only single covalent bonds.
In the Required Alkanes Homologous Series, each member of the series differs from the previous one by a -CH2- unit, resulting in a regular and predictable increase in the number of carbon atoms in the molecule. For example, the series includes compounds such as methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), hexane (C6H14), and so on.
The physical and chemical properties of the Required Alkanes Homologous Series are determined by the size and shape of the molecule, as well as the types of intermolecular forces between molecules. Generally, the boiling point and melting point of alkanes increase as the number of carbon atoms in the molecule increases due to an increase in molecular weight and size.
The Required Alkanes Homologous Series is important in organic chemistry because it allows chemists to predict the properties and behavior of alkanes and their derivatives. It also provides a systematic way of studying and designing new compounds with desired properties by modifying the structure of existing compounds in the series.
Production of Alkanes Homologous series
The production of alkanes homologous series typically involves the extraction, refining, and processing of fossil fuels such as crude oil, natural gas, and coal. These fossil fuels contain hydrocarbons, which are the primary building blocks of alkanes.
The production of alkanes homologous series from crude oil involves a process called fractional distillation. In this process, crude oil is heated and vaporized, and then the resulting vapor is passed through a column or tower containing a series of trays or packing materials. The different hydrocarbons in the vapor have different boiling points and condense at different heights in the tower, allowing them to be separated into different fractions.
The fractions collected from the fractional distillation process include gases such as methane, ethane, and propane, as well as liquids such as butane, pentane, hexane, and higher alkanes. These fractions are further processed to obtain purified alkanes homologous series that meet specific requirements for various applications.
Another method of producing alkanes homologous series is through the Fischer-Tropsch process, which involves the conversion of syngas (a mixture of carbon monoxide and hydrogen) into liquid hydrocarbons, including alkanes. The Fischer-Tropsch process is commonly used to produce synthetic fuels from coal or natural gas, as well as waxes, lubricants, and other chemical products.
In addition to these methods, alkanes homologous series can also be produced through biological processes, such as the anaerobic digestion of organic matter in wastewater treatment plants and landfills, which can produce methane and other gases. The production of alkanes homologous series is an important aspect of the energy industry and the chemical industry, and the demand for these compounds is expected to continue to increase as global energy needs grow.
Case Study on Alkanes Homologous series
One notable case study on alkanes homologous series is their role in the petrochemical industry. Alkanes homologous series, particularly those with longer carbon chains, are the primary components of crude oil and natural gas, which are the raw materials used to produce a wide range of petrochemical products.
For example, the production of polyethylene, one of the most widely used plastics in the world, begins with the extraction and refining of crude oil to obtain ethane gas. Ethane is then converted into ethylene, a key building block for polyethylene, through a process called steam cracking. The ethylene is then polymerized to form long chains of polyethylene, which can be processed into various products such as plastic bags, food packaging, and bottles.
Similarly, the production of other petrochemicals such as propylene, butadiene, and benzene also begins with the extraction and refining of crude oil and natural gas to obtain the necessary alkanes homologous series. These compounds are then processed through various chemical reactions to produce a wide range of products such as rubber, plastics, solvents, and synthetic fibers.
However, the production and use of alkanes homologous series have raised concerns about their impact on the environment and climate change. The burning of fossil fuels releases large amounts of carbon dioxide and other greenhouse gases into the atmosphere, contributing to global warming and climate change. As a result, there is growing interest in the development and use of renewable energy sources and sustainable alternatives to petrochemicals.
Overall, the role of alkanes homologous series in the petrochemical industry highlights their importance as raw materials for the production of a wide range of products. However, their production and use also raise important environmental and societal concerns that need to be addressed.
White paper on Alkanes Homologous series
Introduction
Alkanes homologous series is a group of organic compounds composed of carbon and hydrogen atoms with only single covalent bonds. They are the primary components of crude oil and natural gas and are used in the production of a wide range of petrochemical products. This white paper explores the properties, uses, production, and environmental impact of alkanes homologous series.
Properties of Alkanes Homologous Series
The alkanes homologous series is characterized by a regular and predictable increase in the number of carbon atoms in the molecule, resulting in a systematic increase in molecular weight and size. Each member of the series differs from the previous one by a -CH2- unit, leading to the general formula CnH2n+2.
The physical and chemical properties of alkanes homologous series are determined by the size and shape of the molecule, as well as the types of intermolecular forces between molecules. Generally, the boiling point and melting point of alkanes increase as the number of carbon atoms in the molecule increases due to an increase in molecular weight and size. The higher alkanes also tend to have higher viscosity and lower volatility compared to the lower alkanes.
Uses of Alkanes Homologous Series
The primary use of alkanes homologous series is as a raw material for the production of petrochemicals such as plastics, rubber, solvents, and synthetic fibers. For example, the production of polyethylene, one of the most widely used plastics in the world, begins with the extraction and refining of crude oil to obtain ethane gas. Ethane is then converted into ethylene, a key building block for polyethylene, through a process called steam cracking.
Alkanes homologous series are also used as fuels for heating, transportation, and power generation. Methane, the simplest alkane, is the main component of natural gas and is used as a fuel for heating and cooking. Propane and butane, which have higher boiling points than methane, are used as fuels for heating, cooking, and transportation.
Production of Alkanes Homologous Series
The production of alkanes homologous series typically involves the extraction, refining, and processing of fossil fuels such as crude oil, natural gas, and coal. These fossil fuels contain hydrocarbons, which are the primary building blocks of alkanes.
The production of alkanes homologous series from crude oil involves a process called fractional distillation. In this process, crude oil is heated and vaporized, and then the resulting vapor is passed through a column or tower containing a series of trays or packing materials. The different hydrocarbons in the vapor have different boiling points and condense at different heights in the tower, allowing them to be separated into different fractions.
Another method of producing alkanes homologous series is through the Fischer-Tropsch process, which involves the conversion of syngas (a mixture of carbon monoxide and hydrogen) into liquid hydrocarbons, including alkanes. The Fischer-Tropsch process is commonly used to produce synthetic fuels from coal or natural gas, as well as waxes, lubricants, and other chemical products.
Environmental Impact of Alkanes Homologous Series
The production and use of alkanes homologous series have raised concerns about their impact on the environment and climate change. The burning of fossil fuels releases large amounts of carbon dioxide and other greenhouse gases into the atmosphere, contributing to global warming and climate change. As a result, there is growing interest in the development and use of renewable energy sources and sustainable alternatives to petrochemicals.
Conclusion
In conclusion, alkanes homologous series are an important group of organic compounds used in the production of a wide range of petrochemical products. They are characterized by a predictable increase in molecular weight and size, resulting in systematic changes in their physical and chemical properties. The primary use of alkanes homologous series is as a raw material for the production of plastics, synthetic fibers, and other petrochemical products. The production of alkanes homologous series involves the extraction, refining, and processing of fossil fuels, which has raised concerns about their impact on the environment and climate change. As such, there is growing interest in the development and use of renewable energy sources and sustainable alternatives to petrochemicals.
