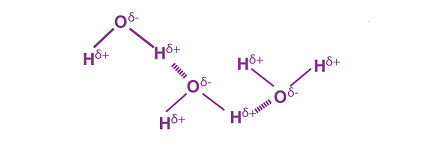
Hydrogen bonding is a type of intermolecular attraction that occurs between a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and a lone pair of electrons on another highly electronegative atom in a nearby molecule. This bonding has several effects:
- Higher boiling and melting points: Hydrogen bonding results in stronger intermolecular forces between molecules, which makes it more difficult to separate them from one another. As a result, substances that exhibit hydrogen bonding tend to have higher boiling and melting points than similar substances that do not exhibit hydrogen bonding.
- Increased viscosity: The stronger intermolecular forces resulting from hydrogen bonding can also increase the viscosity of a substance. This is because the molecules are more tightly packed together, making it more difficult for them to flow past one another.
- Unique physical properties: Hydrogen bonding can give rise to unique physical properties in substances. For example, water has a higher surface tension than other liquids of similar molecular weight due to hydrogen bonding. Additionally, some substances that exhibit hydrogen bonding, such as DNA, have unique structural properties that are critical to their function.
- Solubility: The presence of hydrogen bonding can also affect a substance’s solubility in water. For example, polar substances that exhibit hydrogen bonding tend to be more soluble in water than nonpolar substances.
Overall, hydrogen bonding is an important factor that can significantly influence the physical and chemical properties of substances.
What is Required Basic Principles of Organic Chemistry Hydrogen bonding effects
In organic chemistry, the principles of hydrogen bonding are important to understand because they can influence the reactivity, stability, and properties of organic compounds. Here are some basic principles of organic chemistry hydrogen bonding effects:
- Hydrogen bonding affects the acidity and basicity of organic compounds. For example, a hydrogen bond between a proton and a highly electronegative atom can stabilize the resulting anion and make it more acidic.
- Hydrogen bonding can influence the stability of organic compounds. For example, hydrogen bonds between functional groups in a molecule can stabilize its conformation, making it more thermodynamically stable.
- Hydrogen bonding can affect the solubility and properties of organic compounds in solution. For example, hydrogen bonding between a solvent molecule and a functional group in a solute molecule can increase its solubility in that solvent.
- Hydrogen bonding can also affect the strength and selectivity of intermolecular interactions in organic compounds. For example, hydrogen bonding can lead to the formation of supramolecular structures, such as micelles or aggregates, which can influence the behavior of a mixture of compounds in a variety of ways.
Overall, understanding the principles of hydrogen bonding in organic chemistry is critical for predicting and explaining the behavior of organic compounds in a variety of contexts.
When is Required Basic Principles of Organic Chemistry Hydrogen bonding effects
The principles of hydrogen bonding in organic chemistry are required in a variety of contexts, including:
- Understanding the reactivity and properties of organic compounds: Hydrogen bonding can influence the acidity and basicity of organic compounds, as well as their stability and solubility in different solvents. Therefore, understanding the principles of hydrogen bonding is important for predicting and explaining the reactivity and properties of organic compounds.
- Designing and synthesizing new organic compounds: Hydrogen bonding can play a critical role in determining the structure and properties of organic compounds. Therefore, understanding the principles of hydrogen bonding is important for designing and synthesizing new organic compounds with desired properties.
- Analyzing the behavior of organic compounds in solution: Hydrogen bonding can influence the behavior of organic compounds in solution, including their solubility, viscosity, and other physical properties. Therefore, understanding the principles of hydrogen bonding is important for analyzing the behavior of organic compounds in different solvents and under different conditions.
- Studying the structure and function of biological molecules: Many biological molecules, such as proteins, nucleic acids, and carbohydrates, rely on hydrogen bonding for their structure and function. Therefore, understanding the principles of hydrogen bonding is important for studying the structure and function of these molecules.
Overall, the principles of hydrogen bonding in organic chemistry are required in a wide range of applications and are critical for understanding the behavior of organic compounds in various contexts.
Where is Required Basic Principles of Organic Chemistry Hydrogen bonding effects
The principles of hydrogen bonding in organic chemistry are required in many different fields and applications, including:
- Organic synthesis: The principles of hydrogen bonding are important in designing and synthesizing new organic compounds with desired properties. Understanding how hydrogen bonding can affect the stability and reactivity of different functional groups is important for designing effective synthetic strategies.
- Medicinal chemistry: The principles of hydrogen bonding are important in drug design, where the ability of a molecule to form hydrogen bonds can affect its binding affinity and specificity for a particular target. Understanding how hydrogen bonding affects the structure and properties of different molecules is critical for designing effective drugs.
- Material science: The principles of hydrogen bonding are important in designing and synthesizing new materials with desired properties, such as polymers, surfactants, and nanoparticles. Understanding how hydrogen bonding affects the structure and properties of these materials is critical for developing new technologies and applications.
- Biochemistry: The principles of hydrogen bonding are important in understanding the structure and function of biological molecules, such as proteins, nucleic acids, and carbohydrates. Understanding how hydrogen bonding affects the stability and function of these molecules is critical for understanding biological processes at the molecular level.
Overall, the principles of hydrogen bonding in organic chemistry are important in many different fields and applications where understanding the behavior and properties of organic compounds is critical.
How is Required Basic Principles of Organic Chemistry Hydrogen bonding effects
The principles of hydrogen bonding in organic chemistry are based on the fundamental physical and chemical properties of molecules and their interactions with each other. Some of the key principles include:
- Electronegativity: The ability of an atom to attract electrons towards itself is an important factor in determining whether hydrogen bonding can occur. Atoms with high electronegativity, such as nitrogen, oxygen, and fluorine, can form hydrogen bonds with hydrogen atoms.
- Geometry: The geometry of a molecule can affect the strength and orientation of hydrogen bonding interactions. For example, molecules with linear or planar arrangements of hydrogen bond donor and acceptor groups can form more stable hydrogen bonds than molecules with more complex arrangements.
- Solvent effects: The properties of the solvent can affect the strength and selectivity of hydrogen bonding interactions. For example, a solvent that can donate hydrogen bonds to a solute molecule can stabilize the interaction and increase its strength.
- Functional group effects: Different functional groups in organic compounds can affect the strength and selectivity of hydrogen bonding interactions. For example, carboxylic acids, amides, and alcohols are all functional groups that can participate in hydrogen bonding interactions.
Overall, understanding the basic principles of hydrogen bonding in organic chemistry involves understanding how these factors influence the ability of molecules to form hydrogen bonds, and how hydrogen bonding can affect the properties and behavior of organic compounds in different contexts.
Nomenclature of Basic Principles of Organic Chemistry Hydrogen bonding effects
The nomenclature of organic compounds is an important aspect of organic chemistry, and it provides a systematic way of naming and identifying different types of molecules. The nomenclature of organic compounds typically includes information about their functional groups, their molecular structure, and their stereochemistry. Hydrogen bonding can affect the nomenclature of organic compounds in several ways:
- Naming of functional groups: Some functional groups in organic compounds are known to participate in hydrogen bonding, such as alcohols, carboxylic acids, and amides. Therefore, the presence of these functional groups can be indicated in the name of the compound, such as “ethanol” for a compound with an alcohol functional group.
- Naming of isomers: Isomers are molecules with the same molecular formula but different structures. Hydrogen bonding can affect the structure of isomers, leading to different properties and reactivities. Therefore, the naming of isomers can include information about their hydrogen bonding capabilities, such as “cis” and “trans” isomers for molecules with different orientations of hydrogen bond donor and acceptor groups.
- Stereochemistry: The stereochemistry of organic compounds can be affected by hydrogen bonding, as it can influence the orientation and arrangement of different functional groups. Therefore, the nomenclature of stereoisomers can include information about their hydrogen bonding capabilities, such as “enantiomers” for molecules with opposite stereochemistry at hydrogen bond donor and acceptor groups.
Overall, the nomenclature of organic compounds can be affected by hydrogen bonding in various ways, reflecting its importance in determining the structure and properties of organic molecules.
Case Study on Basic Principles of Organic Chemistry Hydrogen bonding effects
Case Study: The Role of Hydrogen Bonding in Protein Folding
Protein folding is a complex process that involves the self-assembly of amino acid chains into a specific three-dimensional structure. This structure is critical for the function of the protein, as it determines its ability to interact with other molecules and perform specific biological roles. The folding process is guided by a combination of non-covalent interactions, including van der Waals forces, electrostatic interactions, and hydrogen bonding.
Hydrogen bonding plays a critical role in the folding process, as it can stabilize specific structural motifs within the protein, such as alpha-helices and beta-sheets. These motifs are formed by hydrogen bonding interactions between the amide and carbonyl groups of adjacent amino acids, which can lead to the formation of regular secondary structures. In addition, hydrogen bonding can also play a role in the stabilization of tertiary and quaternary structures, by forming interactions between different regions of the protein.
One example of the role of hydrogen bonding in protein folding is the case of the protein lysozyme. Lysozyme is an enzyme that catalyzes the hydrolysis of bacterial cell walls, and it has been extensively studied as a model system for understanding protein folding. Experimental studies have shown that the folding of lysozyme is guided by a combination of hydrophobic interactions and hydrogen bonding.
In particular, hydrogen bonding interactions between the amide and carbonyl groups of adjacent amino acids play a critical role in the formation of the alpha-helices and beta-sheets that make up the protein’s secondary structure. In addition, hydrogen bonding interactions between different regions of the protein can lead to the formation of a specific tertiary structure, which is critical for the protein’s catalytic activity.
Overall, the role of hydrogen bonding in protein folding highlights the importance of non-covalent interactions in guiding the self-assembly of biomolecules into specific structures. Understanding the principles of hydrogen bonding and its effects on protein structure and stability is critical for developing new approaches to protein engineering and drug design.
White paper on Basic Principles of Organic Chemistry Hydrogen bonding effects
Introduction:
Hydrogen bonding is a type of intermolecular interaction that plays a critical role in organic chemistry. It involves the attraction between a hydrogen atom bonded to an electronegative atom, such as nitrogen, oxygen, or fluorine, and another electronegative atom in a different molecule. Hydrogen bonding can lead to the formation of specific structures and properties in organic compounds, and it is important in many biological and chemical processes. This white paper will provide an overview of the basic principles of hydrogen bonding in organic chemistry and its effects on the properties and behavior of organic compounds.
Basic Principles of Hydrogen Bonding:
The principles of hydrogen bonding in organic chemistry are based on several key factors, including electronegativity, geometry, solvent effects, and functional group effects. Electronegative atoms, such as nitrogen, oxygen, and fluorine, are able to attract electrons towards themselves, creating a partial negative charge. This creates a region of positive charge on the hydrogen atom bonded to the electronegative atom, which can then form a hydrogen bond with another electronegative atom in a different molecule.
The geometry of a molecule can also affect the strength and orientation of hydrogen bonding interactions. Linear or planar arrangements of hydrogen bond donor and acceptor groups can form more stable hydrogen bonds than more complex arrangements. Solvent effects can also influence the strength and selectivity of hydrogen bonding interactions. For example, a solvent that can donate hydrogen bonds to a solute molecule can stabilize the interaction and increase its strength.
Functional groups in organic compounds can also affect the strength and selectivity of hydrogen bonding interactions. For example, carboxylic acids, amides, and alcohols are all functional groups that can participate in hydrogen bonding interactions.
Effects of Hydrogen Bonding on Organic Compounds:
Hydrogen bonding can have a range of effects on the properties and behavior of organic compounds, depending on the specific context. For example, hydrogen bonding can affect the boiling point and solubility of organic compounds, as it can lead to the formation of stable structures in solution. In addition, hydrogen bonding can affect the reactivity and selectivity of organic reactions, as it can influence the orientation and arrangement of functional groups.
In biological systems, hydrogen bonding plays a critical role in the structure and function of biomolecules such as proteins and nucleic acids. Hydrogen bonding interactions between amino acid residues in proteins can stabilize specific structural motifs, such as alpha-helices and beta-sheets, which are critical for the protein’s function. Hydrogen bonding can also play a role in the recognition and binding of molecules, such as in the formation of enzyme-substrate complexes.
Conclusion:
Hydrogen bonding is a fundamental concept in organic chemistry that has important implications for the properties and behavior of organic compounds. Understanding the basic principles of hydrogen bonding and its effects on organic compounds is critical for developing new approaches to organic synthesis, drug design, and biomolecular engineering. The ability to manipulate and control hydrogen bonding interactions is a key area of research in organic chemistry, with potential applications in fields such as materials science, nanotechnology, and biotechnology.
