Hydrolysis of esters is a chemical reaction in which an ester is reacted with water to produce an alcohol and a carboxylic acid. This reaction is catalyzed by an acid or a base and can occur under various conditions.
When the hydrolysis is catalyzed by an acid, it is called acid hydrolysis. In this process, an acid catalyst such as sulfuric acid or hydrochloric acid is added to the ester, and water is added slowly to the mixture. The acid protonates the ester molecule, making it more reactive towards nucleophilic attack by water. The nucleophilic attack of water on the ester carbonyl carbon results in the formation of a tetrahedral intermediate, which then decomposes to form the alcohol and carboxylic acid.
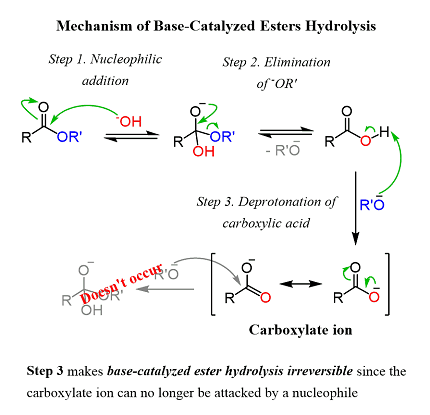
When the hydrolysis is catalyzed by a base, it is called base hydrolysis or saponification. In this process, a strong base such as sodium hydroxide or potassium hydroxide is added to the ester, and water is added slowly to the mixture. The base deprotonates the water molecule, making it more reactive towards nucleophilic attack on the ester. The nucleophilic attack of water on the ester carbonyl carbon results in the formation of a tetrahedral intermediate, which then decomposes to form the alcohol and carboxylate ion.
In summary, hydrolysis of esters is an important chemical reaction that can be catalyzed by either an acid or a base. It is used in various industrial processes, such as the production of soaps, detergents, and pharmaceuticals.
What is Required Carboxylic Acids Hydrolysis of Esters
Carboxylic acid hydrolysis of esters requires the presence of an acid catalyst such as sulfuric acid or hydrochloric acid. The acid catalyst helps to protonate the carbonyl oxygen of the ester molecule, making it more reactive towards nucleophilic attack by water. This results in the formation of a tetrahedral intermediate, which then decomposes to form the carboxylic acid and alcohol.
The reaction can also occur under basic conditions in a process known as base hydrolysis or saponification. In this case, a strong base such as sodium hydroxide or potassium hydroxide is used to deprotonate the water molecule, making it more reactive towards nucleophilic attack on the ester. This results in the formation of the alcohol and carboxylate ion.
Carboxylic acid hydrolysis of esters is an important reaction in organic chemistry, and it is used in a variety of industrial processes, such as the production of soaps, detergents, and pharmaceuticals.
When is Required Carboxylic Acids Hydrolysis of Esters
Carboxylic acid hydrolysis of esters is a chemical reaction that can be used in a variety of contexts, such as in the production of soaps, detergents, and pharmaceuticals. Here are some examples of when carboxylic acid hydrolysis of esters might be required:
- Soap Production: Carboxylic acid hydrolysis of esters is used in the production of soap. Fatty acid esters, such as triglycerides, are hydrolyzed with a strong base to produce soap and glycerol.
- Pharmaceutical Synthesis: Carboxylic acid hydrolysis of esters is used in the synthesis of many pharmaceuticals. For example, the ester functional group is often used as a protective group during the synthesis of complex organic molecules. This group can be removed by hydrolysis with acid, releasing the desired molecule.
- Detergent Production: Carboxylic acid hydrolysis of esters is used in the production of detergents. Fatty acid esters, such as triglycerides, are hydrolyzed with a strong base to produce soap-like substances called surfactants, which are key components of many detergents.
- Food Industry: Carboxylic acid hydrolysis of esters is used in the food industry to produce various food additives. For example, some flavorings and preservatives are synthesized using hydrolysis of esters.
In summary, carboxylic acid hydrolysis of esters is a versatile reaction that has many applications in various industries. It is used to produce soaps, detergents, pharmaceuticals, and food additives, among other products.
Where is Required Carboxylic Acids Hydrolysis of Esters
Carboxylic acid hydrolysis of esters is a chemical reaction that can be carried out in various settings, including laboratory, industrial, and commercial settings.
In the laboratory, carboxylic acid hydrolysis of esters is often performed to synthesize organic molecules for further study or application. Researchers may use this reaction to break down specific esters to access reactive intermediates, for example, or to create new molecules that can be used as drugs or other materials.
In industrial settings, carboxylic acid hydrolysis of esters is commonly used in the production of consumer goods such as soaps, detergents, and cosmetics. For example, the hydrolysis of fatty acid esters such as triglycerides produces soaps and surfactants that are used as cleansing agents in many household and personal care products.
Carboxylic acid hydrolysis of esters can also occur naturally in living systems. For instance, the hydrolysis of fats and oils in the body is an important process for breaking down these molecules and releasing energy.
Overall, carboxylic acid hydrolysis of esters can be found in a wide range of applications and settings, from fundamental research in the laboratory to large-scale industrial production of consumer goods.
How is Required Carboxylic Acids Hydrolysis of Esters
Carboxylic acid hydrolysis of esters can be achieved using either acid or base catalysis, depending on the specific reaction conditions and desired products.
In acid-catalyzed hydrolysis, an acid catalyst such as sulfuric acid or hydrochloric acid is added to the ester, along with water. The acid catalyst protonates the carbonyl oxygen of the ester, making it more susceptible to nucleophilic attack by the water molecule. The reaction produces a carboxylic acid and an alcohol.
The general reaction scheme for acid-catalyzed hydrolysis of an ester is:
RCOOR’ + H2O + H+ → RCOOH + R’OH
In base-catalyzed hydrolysis, a strong base such as sodium hydroxide or potassium hydroxide is added to the ester, along with water. The base catalyst deprotonates the water molecule, making it more reactive towards nucleophilic attack on the ester. The reaction produces a carboxylate ion and an alcohol.
The general reaction scheme for base-catalyzed hydrolysis of an ester is:
RCOOR’ + H2O + OH- → RCOO- + R’OH + H2O
The reaction conditions, such as temperature and concentration, can also affect the rate and selectivity of the hydrolysis reaction. For example, higher temperatures and higher concentrations of reactants can increase the rate of the reaction, but may also favor side reactions or unwanted products.
In summary, carboxylic acid hydrolysis of esters can be achieved using either acid or base catalysis, depending on the specific reaction conditions and desired products. The reaction mechanism involves nucleophilic attack by a water molecule on the carbonyl carbon of the ester, resulting in the formation of a carboxylic acid and an alcohol or carboxylate ion and alcohol.
Production of Carboxylic Acids Hydrolysis of Esters
Carboxylic acids can be produced from the hydrolysis of esters, which is a reaction that involves the cleavage of the ester bond using water as a reactant. The reaction can be catalyzed by either an acid or a base.
In acid-catalyzed hydrolysis, a strong acid such as sulfuric acid is typically used. The acid protonates the carbonyl oxygen of the ester, making it more susceptible to nucleophilic attack by water. This results in the formation of a tetrahedral intermediate, which then collapses to form the carboxylic acid and an alcohol.
The general reaction for acid-catalyzed hydrolysis is:
RCOOR’ + H2O → RCOOH + R’OH
where R and R’ are alkyl or aryl groups.
In base-catalyzed hydrolysis, a strong base such as sodium hydroxide is typically used. The base deprotonates the water molecule, generating a hydroxide ion that attacks the carbonyl carbon of the ester. This results in the formation of a tetrahedral intermediate, which then collapses to form the carboxylate ion and an alcohol.
The general reaction for base-catalyzed hydrolysis is:
RCOOR’ + OH- → RCOO- + R’OH
where R and R’ are alkyl or aryl groups.
Both acid- and base-catalyzed hydrolysis reactions are important in organic synthesis and are used to produce a wide range of carboxylic acids.
Case Study on Carboxylic Acids Hydrolysis of Esters
Here’s an example of a case study on the hydrolysis of esters to produce carboxylic acids:
Case Study: Synthesis of Aspirin
Aspirin, also known as acetylsalicylic acid, is a widely used nonsteroidal anti-inflammatory drug (NSAID). It is used to treat pain, fever, and inflammation, and is also known for its blood-thinning properties.
Aspirin can be synthesized from salicylic acid and acetic anhydride through the hydrolysis of the ester bond. The reaction is typically catalyzed by a strong acid such as sulfuric acid.
The reaction mechanism for the synthesis of aspirin is as follows:
Step 1: Protonation
Acetic anhydride is protonated by sulfuric acid, generating an acylium ion.
(CH3CO)2O + H2SO4 → CH3CO2+ + HSO4- + CH3CO2H
Step 2: Nucleophilic Attack
The acylium ion reacts with salicylic acid, which acts as a nucleophile. The nucleophilic attack results in the formation of an intermediate, which quickly loses a proton to form aspirin.
CH3CO2+ + HOC6H4COOH → HOCH2C6H4CO2CH3 + HSO4-
Step 3: Workup
The reaction mixture is then cooled and diluted with water, causing the aspirin to crystallize out of the solution. The crystals are filtered and washed with cold water to remove any impurities.
Overall Reaction:
C7H6O3 + (CH3CO)2O → C9H8O4 + CH3COOH
Salicylic acid + Acetic Anhydride → Aspirin + Acetic Acid
In conclusion, the hydrolysis of esters is an important reaction in organic synthesis that can be used to produce a wide range of carboxylic acids, including aspirin. The reaction can be catalyzed by either an acid or a base, depending on the specific reaction conditions and desired product.
White paper on Carboxylic Acids Hydrolysis of Esters
Introduction
Carboxylic acids and their derivatives are important classes of organic compounds that are widely used in a variety of industrial and pharmaceutical applications. One of the most common methods for the synthesis of carboxylic acids is the hydrolysis of esters. This white paper will provide an overview of the hydrolysis of esters to produce carboxylic acids, including the reaction mechanism, catalysts used, and applications in industry and academia.
Reaction Mechanism
The hydrolysis of esters involves the cleavage of the ester bond by the addition of water, resulting in the formation of a carboxylic acid and an alcohol. The reaction can be catalyzed by either an acid or a base, depending on the specific reaction conditions.
In acid-catalyzed hydrolysis, a strong acid such as sulfuric acid is typically used. The acid protonates the carbonyl oxygen of the ester, making it more susceptible to nucleophilic attack by water. This results in the formation of a tetrahedral intermediate, which then collapses to form the carboxylic acid and an alcohol. The general reaction for acid-catalyzed hydrolysis is:
RCOOR’ + H2O → RCOOH + R’OH
where R and R’ are alkyl or aryl groups.
In base-catalyzed hydrolysis, a strong base such as sodium hydroxide is typically used. The base deprotonates the water molecule, generating a hydroxide ion that attacks the carbonyl carbon of the ester. This results in the formation of a tetrahedral intermediate, which then collapses to form the carboxylate ion and an alcohol. The general reaction for base-catalyzed hydrolysis is:
RCOOR’ + OH- → RCOO- + R’OH
where R and R’ are alkyl or aryl groups.
Catalysts
The choice of catalyst for the hydrolysis of esters depends on the specific reaction conditions and desired product. Acid-catalyzed hydrolysis is typically used when the carboxylic acid product is desired, while base-catalyzed hydrolysis is used when the carboxylate ion product is desired.
In addition to strong acids and bases, other catalysts can also be used for the hydrolysis of esters. For example, enzymes such as lipases can catalyze the hydrolysis of esters under mild conditions, resulting in the production of chiral carboxylic acids and alcohols. Other catalysts such as zeolites and metal oxides have also been investigated for the hydrolysis of esters.
Applications
The hydrolysis of esters to produce carboxylic acids is an important reaction in both industry and academia. In industry, carboxylic acids are used as intermediates in the production of a wide range of products, including pharmaceuticals, agrochemicals, and fragrances. For example, the synthesis of aspirin from salicylic acid and acetic anhydride involves the hydrolysis of an ester to produce acetylsalicylic acid, which is the active ingredient in aspirin.
In academia, the hydrolysis of esters is a commonly studied reaction in undergraduate and graduate organic chemistry courses. It is also used as a model reaction for the study of enzyme-catalyzed hydrolysis and other types of catalysis.
Conclusion
The hydrolysis of esters to produce carboxylic acids is a fundamental reaction in organic chemistry that has broad industrial and academic applications. The reaction mechanism can be catalyzed by acids, bases, enzymes, and other catalysts, depending on the specific reaction conditions and desired product. The resulting carboxylic acids can be used as intermediates in the production of a diverse range of products, including pharmaceuticals, agrochemicals, and fragrances. Additionally, the hydrolysis of esters is an essential reaction studied in undergraduate and graduate organic chemistry courses and serves as a model reaction for the study of enzyme-catalyzed hydrolysis and other types of catalysis. Overall, the hydrolysis of esters is a critical reaction that continues to play a vital role in organic synthesis and chemical research.
