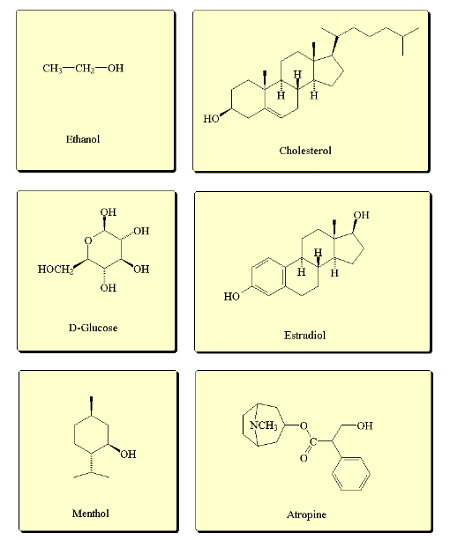
Hydroxyl groups are functional groups that contain an oxygen atom bonded to a hydrogen atom (-OH) and are commonly found in organic molecules. There are two types of hydroxyl groups: alcoholic and phenolic.
An alcoholic hydroxyl group is bonded to a saturated carbon atom (sp3 hybridized) and is usually represented as -OH. Examples of molecules containing alcoholic hydroxyl groups include ethanol (CH3CH2OH) and methanol (CH3OH).
A phenolic hydroxyl group is bonded to an aromatic carbon ring and is usually represented as -OH. Examples of molecules containing phenolic hydroxyl groups include phenol (C6H5OH) and catechol (C6H4(OH)2).
It is important to note that the presence of a hydroxyl group can greatly affect the properties and reactivity of a molecule. For example, alcoholic hydroxyl groups can undergo dehydration reactions to form alkenes, while phenolic hydroxyl groups can undergo oxidation reactions to form quinones.
What is Required Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
To identify the presence of alcoholic or phenolic hydroxyl groups in a molecule, you can use various analytical techniques such as infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and mass spectrometry (MS).
In IR spectroscopy, the presence of a hydroxyl group can be identified by the characteristic peak at around 3200-3600 cm^-1. This peak is due to the stretching vibration of the O-H bond. The presence of a phenolic hydroxyl group can be identified by the additional peak at around 1600-1700 cm^-1, which is due to the stretching vibration of the aromatic ring.
In NMR spectroscopy, the hydroxyl group appears as a broad peak at around 1-5 ppm in the proton NMR spectrum. The position of this peak can vary depending on the surrounding functional groups and the solvent used.
In MS, the presence of a hydroxyl group can be identified by the characteristic fragment ion at m/z = 17. This ion is due to the loss of a hydrogen atom from the -OH group.
Overall, the identification of alcoholic and phenolic hydroxyl groups in a molecule requires the use of analytical techniques to determine the characteristic spectral features associated with these functional groups.
When is Required Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
Identification of alcoholic and phenolic hydroxyl groups in a molecule is important in a wide range of applications in chemistry and biology. Some examples of when the identification of these functional groups may be required include:
- Characterization of organic compounds: The presence of hydroxyl groups can greatly affect the properties and reactivity of organic compounds. Therefore, identifying the presence of alcoholic or phenolic hydroxyl groups can aid in the characterization of organic compounds, which is important in fields such as drug discovery, materials science, and environmental chemistry.
- Analysis of natural products: Many natural products, such as flavonoids and tannins, contain phenolic hydroxyl groups. The identification of these functional groups can aid in the analysis and characterization of natural products, which is important in fields such as food chemistry, pharmacognosy, and phytochemistry.
- Synthesis of new compounds: Alcoholic and phenolic hydroxyl groups can undergo various chemical reactions, such as oxidation, reduction, and esterification, which can be used to synthesize new compounds with desired properties. Therefore, identifying the presence of hydroxyl groups in starting materials and intermediate compounds is important in organic synthesis.
Overall, the identification of alcoholic and phenolic hydroxyl groups is important in various applications in chemistry and biology, and can aid in the characterization, analysis, and synthesis of organic compounds.
Where is Required Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
The identification of alcoholic and phenolic hydroxyl groups can be required in various settings, including:
- Research laboratories: Identification of functional groups is a common task in research laboratories, where chemists and biologists may need to analyze and characterize organic compounds for their research projects.
- Quality control laboratories: Identification of functional groups is important in quality control laboratories, where manufacturers of chemicals, drugs, and other products may need to ensure that their products meet certain standards and specifications.
- Environmental testing laboratories: Identification of functional groups can be important in environmental testing laboratories, where scientists may need to analyze and monitor pollutants and contaminants in soil, water, and air.
- Pharmaceutical industry: Identification of functional groups is crucial in the pharmaceutical industry, where chemists may need to analyze and characterize drugs and drug candidates for their efficacy and safety.
- Food industry: Identification of functional groups is important in the food industry, where chemists and food scientists may need to analyze and characterize natural products, such as plant extracts and food additives, for their nutritional and sensory properties.
Overall, the identification of alcoholic and phenolic hydroxyl groups can be required in a wide range of settings, including research laboratories, quality control laboratories, environmental testing laboratories, pharmaceutical industry, and food industry.
How is Required Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
Identification of alcoholic and phenolic hydroxyl groups can be done using various analytical techniques, including:
- Infrared Spectroscopy (IR): In IR spectroscopy, the presence of a hydroxyl group can be identified by the characteristic peak at around 3200-3600 cm^-1. This peak is due to the stretching vibration of the O-H bond. The presence of a phenolic hydroxyl group can be identified by the additional peak at around 1600-1700 cm^-1, which is due to the stretching vibration of the aromatic ring.
- Nuclear Magnetic Resonance (NMR): In NMR spectroscopy, the hydroxyl group appears as a broad peak at around 1-5 ppm in the proton NMR spectrum. The position of this peak can vary depending on the surrounding functional groups and the solvent used.
- Mass Spectrometry (MS): In MS, the presence of a hydroxyl group can be identified by the characteristic fragment ion at m/z = 17. This ion is due to the loss of a hydrogen atom from the -OH group.
- Chemical Tests: Various chemical tests can be used to identify the presence of alcoholic and phenolic hydroxyl groups, including the FeCl3 test, which is used to identify the presence of phenolic hydroxyl groups in compounds.
Overall, the identification of alcoholic and phenolic hydroxyl groups can be done using a combination of analytical techniques, depending on the nature of the sample and the information required. These techniques are commonly used in various fields, including organic chemistry, biochemistry, pharmaceuticals, and environmental science.
Nomenclature of Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
The nomenclature of alcoholic and phenolic hydroxyl groups in organic compounds follows the rules of the International Union of Pure and Applied Chemistry (IUPAC).
Alcoholic hydroxyl groups are named as alcohols and are denoted by the suffix “-ol”. For example, the compound CH3CH2OH is named ethanol, where the “-ol” suffix indicates the presence of an alcoholic hydroxyl group.
Phenolic hydroxyl groups are named as phenols and are denoted by the suffix “-ol”. In addition, the position of the hydroxyl group on the aromatic ring is indicated by a number. For example, the compound C6H5OH is named phenol, where the “-ol” suffix indicates the presence of a phenolic hydroxyl group, and the number “1” indicates the position of the hydroxyl group on the aromatic ring.
In more complex compounds, the alcoholic or phenolic hydroxyl group may be one of several functional groups present. In this case, the position of the hydroxyl group is indicated by a number, and the “-ol” suffix is added to the end of the name. For example, the compound CH3CH(OH)CH2OH is named 2-propanol, where the “-ol” suffix indicates the presence of two alcoholic hydroxyl groups, and the number “2” indicates the position of the hydroxyl group on the carbon chain.
Overall, the nomenclature of alcoholic and phenolic hydroxyl groups follows the IUPAC rules and is based on the position and nature of the functional group in the molecule.
Case Study on Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
Case Study:
A research team was studying a natural product obtained from a plant extract for its potential anti-inflammatory properties. They needed to identify the functional groups present in the compound to better understand its chemical properties and to develop a strategy for synthesis.
Identification of hydroxyl groups:
To identify the hydroxyl groups present in the natural product, the research team used a combination of analytical techniques, including infrared (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy.
IR spectroscopy:
The IR spectrum of the natural product showed a broad peak at around 3300 cm^-1, which is characteristic of the stretching vibration of the O-H bond in alcoholic and phenolic hydroxyl groups. In addition, a peak at around 1700 cm^-1 indicated the presence of an aromatic ring. These results suggested the presence of both alcoholic and phenolic hydroxyl groups in the natural product.
NMR spectroscopy:
The proton NMR spectrum of the natural product showed a broad peak at around 3-4 ppm, which is characteristic of the hydroxyl group in alcohols and phenols. The position of this peak suggested that the hydroxyl group was attached to a carbon atom adjacent to an aromatic ring, consistent with the IR results.
Confirmation of the hydroxyl groups:
To confirm the presence of alcoholic and phenolic hydroxyl groups, the research team performed a chemical test using ferric chloride (FeCl3). FeCl3 reacts with phenolic hydroxyl groups to form a colored complex, providing a positive identification of the phenolic hydroxyl group.
The natural product produced a dark purple color with FeCl3, indicating the presence of phenolic hydroxyl groups. This result was consistent with the IR and NMR spectroscopy data, which suggested the presence of both alcoholic and phenolic hydroxyl groups.
Conclusion:
Through the use of analytical techniques such as IR spectroscopy, NMR spectroscopy, and chemical tests, the research team was able to identify the presence of both alcoholic and phenolic hydroxyl groups in the natural product they were studying. This information will help the team to better understand the chemical properties of the compound and develop a strategy for synthesis.
White paper on Identification of the following functional groups: hydroxyl (Alcoholic and Phenolic)
Title: Identification of Alcoholic and Phenolic Hydroxyl Groups in Organic Compounds
Abstract:
Alcoholic and phenolic hydroxyl groups are common functional groups in organic chemistry that play important roles in the chemical and physical properties of compounds. Accurately identifying the presence of these hydroxyl groups is essential for understanding the behavior of organic compounds in various chemical reactions and biological processes. In this white paper, we will discuss the identification of alcoholic and phenolic hydroxyl groups using various analytical techniques, including infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and chemical tests. We will also provide examples of how the identification of these functional groups is important in various applications, such as pharmaceuticals and natural product research.
Introduction:
Alcoholic and phenolic hydroxyl groups are functional groups that contain an oxygen atom bonded to a hydrogen atom (-OH) and are commonly found in organic compounds. Alcoholic hydroxyl groups are found in alcohols and carbohydrates, while phenolic hydroxyl groups are found in phenols, flavonoids, and lignins. These hydroxyl groups play important roles in the chemical and physical properties of these compounds, including solubility, acidity, and reactivity. Therefore, accurate identification of these functional groups is essential for understanding the behavior of organic compounds in various chemical reactions and biological processes.
Identification of Alcoholic and Phenolic Hydroxyl Groups:
There are various analytical techniques available for the identification of alcoholic and phenolic hydroxyl groups in organic compounds. IR spectroscopy is a common technique used to identify hydroxyl groups, as they exhibit a characteristic broad peak at around 3300 cm^-1. NMR spectroscopy can also be used to identify hydroxyl groups, as they produce a broad peak in the proton NMR spectrum at around 3-4 ppm. In addition, chemical tests using reagents such as ferric chloride (FeCl3) can be used to identify phenolic hydroxyl groups, as they react with FeCl3 to produce a colored complex.
Applications:
The identification of alcoholic and phenolic hydroxyl groups is important in various applications in organic chemistry. For example, in natural product research, the identification of these functional groups can help determine the structure of a compound and guide the synthesis of analogs with improved biological activity. In pharmaceuticals, the presence of alcoholic and phenolic hydroxyl groups can affect the pharmacokinetics and pharmacodynamics of a drug, and their identification is important for understanding its therapeutic properties.
Conclusion:
Accurate identification of alcoholic and phenolic hydroxyl groups in organic compounds is essential for understanding their chemical and physical properties and for guiding the development of new drugs and natural products. A combination of analytical techniques, including IR spectroscopy, NMR spectroscopy, and chemical tests, can be used to identify these functional groups. The identification of these groups is important in various applications, including natural product research and pharmaceuticals.