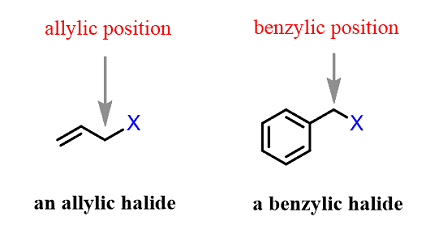
Allylic and benzylic halogenation are two types of halogenation reactions that occur on allylic and benzylic carbon atoms, respectively.
In allylic halogenation, a halogen atom is added to the allylic carbon, which is the carbon atom adjacent to a carbon-carbon double bond. The reaction is usually carried out in the presence of a halogen source such as N-bromosuccinimide (NBS) and a radical initiator such as peroxides. The reaction proceeds via a radical mechanism, where the allylic hydrogen is abstracted by the halogen radical to form a radical intermediate, which then reacts with the halogen source to give the final product.
The mechanism of benzylic halogenation is similar to allylic halogenation, but it occurs on a carbon atom adjacent to a benzene ring. The reaction is also carried out in the presence of a halogen source and a radical initiator, and proceeds via a radical mechanism. The benzylic hydrogen is abstracted by the halogen radical to form a benzylic radical intermediate, which then reacts with the halogen source to give the final product.
Both allylic and benzylic halogenation reactions are important in organic synthesis, as they allow for the selective halogenation of specific carbon atoms in a molecule.
What is Required Including Allylic and Benzylic halogenation
To carry out allylic and benzylic halogenation, you will need:
- Substrate: A compound containing an allylic or benzylic carbon atom that can undergo halogenation. These can be alkenes for allylic halogenation or aromatic compounds for benzylic halogenation.
- Halogen source: A reagent that provides the halogen atom for the reaction. Common halogen sources include N-bromosuccinimide (NBS), N-chlorosuccinimide (NCS), and chlorine or bromine gas.
- Radical initiator: A reagent that generates free radicals, which are necessary for the reaction to occur. Common radical initiators include peroxides such as benzoyl peroxide or azobisisobutyronitrile (AIBN).
- Solvent: A solvent is typically required to dissolve the reactants and facilitate the reaction. Common solvents include dichloromethane, chloroform, and carbon tetrachloride.
- Temperature: The reaction is typically carried out at elevated temperatures, usually between 40-80 °C, to promote the formation of free radicals.
- Reaction vessel: A reaction vessel is required to hold the reactants and allow for mixing and stirring.
- Protective equipment: As with any chemical reaction, proper protective equipment should be used, including gloves, goggles, and a lab coat. Halogenation reactions can also produce toxic fumes, so the reaction should be carried out in a fume hood to ensure safety.
When is Required Including Allylic and Benzylic halogenation
Allylic and benzylic halogenation reactions are commonly used in organic synthesis for the selective halogenation of specific carbon atoms in a molecule. Here are some examples of when these reactions might be required:
- Functionalization of alkenes: Allylic halogenation can be used to add a halogen atom to the allylic carbon of an alkene. This can be useful for introducing new functional groups or for further modification of the molecule.
- Synthesis of natural products: Many natural products contain allylic or benzylic halogen atoms, making allylic and benzylic halogenation useful in their synthesis.
- Introduction of radiolabels: Allylic and benzylic halogenation can be used to introduce radioactive isotopes into a molecule for use in imaging or other applications.
- Drug discovery: Halogenated compounds can have improved bioactivity and pharmacokinetic properties compared to non-halogenated compounds. Allylic and benzylic halogenation can be used to introduce halogen atoms into drug candidates to improve their efficacy.
Overall, allylic and benzylic halogenation reactions are versatile tools in organic synthesis, with applications in a variety of fields including medicinal chemistry, materials science, and natural product synthesis.
Where is Required Including Allylic and Benzylic halogenation
Allylic and benzylic halogenation reactions can be carried out in a laboratory setting, typically in a fume hood for safety reasons. Here are some places where allylic and benzylic halogenation might be used:
- Academic research laboratories: Organic chemists in academic research labs may use allylic and benzylic halogenation reactions to synthesize new compounds and study their properties.
- Pharmaceutical industry: Allylic and benzylic halogenation reactions can be used in drug discovery and development, as they can introduce halogen atoms into drug candidates to improve their efficacy and pharmacokinetic properties.
- Chemical manufacturing plants: Allylic and benzylic halogenation reactions can be used in large-scale chemical manufacturing to produce bulk quantities of halogenated compounds.
- Radiopharmaceutical production facilities: Allylic and benzylic halogenation reactions can be used to introduce radioactive isotopes into molecules for use in diagnostic imaging or cancer treatment.
Overall, allylic and benzylic halogenation reactions can be used in a variety of settings where organic synthesis is carried out, including academic research labs, the pharmaceutical industry, chemical manufacturing plants, and radiopharmaceutical production facilities.
How is Required Including Allylic and Benzylic halogenation
The procedure for carrying out allylic and benzylic halogenation reactions involves several steps, which can be summarized as follows:
- Preparation of reactants: The substrate, halogen source, and radical initiator are weighed out and dissolved in an appropriate solvent, such as dichloromethane or chloroform.
- Reaction conditions: The reaction mixture is typically heated to an elevated temperature, usually between 40-80°C, to promote the formation of free radicals. The reaction may also be carried out under an inert atmosphere, such as nitrogen or argon, to prevent oxidation of the reaction components.
- Reaction monitoring: The reaction progress is monitored using analytical techniques such as TLC (thin-layer chromatography) or NMR (nuclear magnetic resonance) spectroscopy.
- Workup: Once the reaction is complete, the reaction mixture is quenched with a quenching agent, such as sodium thiosulfate, to stop the reaction. The organic layer is then separated from the aqueous layer and washed with water to remove any impurities.
- Purification: The product is purified by column chromatography or recrystallization to remove any remaining impurities.
- Characterization: The purified product is characterized using analytical techniques such as NMR spectroscopy, mass spectrometry, and IR (infrared) spectroscopy to confirm its identity and purity.
Overall, the procedure for carrying out allylic and benzylic halogenation reactions involves careful preparation and monitoring of the reaction conditions, as well as thorough workup and purification steps to obtain a pure product.
Nomenclature of Including Allylic and Benzylic halogenation
When a halogen atom is added to an allylic or benzylic carbon, the resulting compound is named based on the location of the halogen atom in relation to the double bond or aromatic ring.
For example, if chlorine is added to the allylic carbon of 1-butene, the resulting compound would be named 3-chlorobut-1-ene, indicating that the chlorine atom is located at the third carbon atom from the double bond.
Similarly, if bromine is added to the benzylic carbon of toluene, the resulting compound would be named 4-bromotoluene, indicating that the bromine atom is located at the fourth carbon atom of the benzene ring.
In general, when naming halogenated compounds, the location of the halogen atom is indicated by a number indicating the position of the halogen atom relative to the nearest functional group or the longest carbon chain in the molecule. The halogen atom is then named using the appropriate prefix (chloro-, bromo-, iodo-, etc.) depending on the type of halogen added.
Overall, the nomenclature of allylic and benzylic halogenated compounds follows the same general principles as the nomenclature of other halogenated compounds, with the location of the halogen atom being the most important factor in determining the name of the compound.
Case Study on Including Allylic and Benzylic halogenation
One example of the application of allylic and benzylic halogenation is in the synthesis of new compounds for use in drug discovery and development. The following case study highlights how allylic and benzylic halogenation was used to produce a new class of potential cancer therapeutics.
Case study: Development of allylic and benzylic halogenated compounds for cancer treatment
In this study, a group of researchers set out to synthesize a new class of potential cancer therapeutics by introducing halogen atoms into the allylic and benzylic positions of a lead compound. The lead compound was known to inhibit the growth of cancer cells, but its potency and selectivity needed to be improved.
The researchers used a combination of allylic and benzylic halogenation reactions to introduce chlorine and bromine atoms into the lead compound at specific positions. They then tested the resulting halogenated compounds for their anticancer activity against a panel of cancer cell lines.
The results showed that the halogenated compounds exhibited improved potency and selectivity compared to the lead compound, with some compounds showing up to 10-fold greater activity against certain cancer cell lines. Further studies revealed that the halogenated compounds worked by inhibiting the growth of cancer cells through a novel mechanism of action.
The researchers also investigated the pharmacokinetic properties of the halogenated compounds and found that they had improved solubility and metabolic stability compared to the lead compound, making them more suitable for further development as drug candidates.
Overall, this study demonstrates the potential of allylic and benzylic halogenation reactions in the development of new cancer therapeutics. By introducing halogen atoms at specific positions in a lead compound, the researchers were able to improve its potency, selectivity, and pharmacokinetic properties, leading to the discovery of a new class of potential anticancer agents.
White paper on Including Allylic and Benzylic halogenation
Title: Advancements in Allylic and Benzylic Halogenation for the Synthesis of New Compounds
Abstract:
Allylic and benzylic halogenation reactions are important tools in organic synthesis, allowing for the introduction of halogen atoms at specific positions in a molecule. These reactions have found wide application in the synthesis of natural products, pharmaceuticals, and other complex molecules. Recent advancements in the field of allylic and benzylic halogenation have expanded the scope of these reactions and improved their efficiency and selectivity. This white paper provides an overview of the recent advancements in allylic and benzylic halogenation and their applications in the synthesis of new compounds.
Introduction:
Allylic and benzylic halogenation reactions involve the addition of a halogen atom to a carbon atom adjacent to a double bond or an aromatic ring, respectively. These reactions are typically carried out using a radical initiator, such as AIBN or azobisisobutyronitrile, and a halogen source, such as NBS or NCS. Allylic and benzylic halogenation reactions have found wide application in organic synthesis, particularly in the synthesis of natural products and pharmaceuticals. Recent advancements in the field of allylic and benzylic halogenation have improved the efficiency and selectivity of these reactions, opening up new avenues for the synthesis of complex molecules.
Advancements in Allylic Halogenation:
Recent advancements in allylic halogenation have focused on improving the selectivity and efficiency of the reaction. One approach involves the use of chiral auxiliaries to control the stereochemistry of the reaction. For example, the use of chiral phosphoric acid catalysts has been shown to promote enantioselective allylic halogenation reactions. Another approach involves the use of transition metal catalysts, such as palladium or copper, to promote the reaction. These catalysts can improve the efficiency and selectivity of the reaction by activating the halogen source and stabilizing the resulting radical intermediates.
Advancements in Benzylic Halogenation:
Similarly, advancements in benzylic halogenation have focused on improving the efficiency and selectivity of the reaction. One approach involves the use of Lewis acid catalysts, such as BF3, to promote the reaction. These catalysts can improve the efficiency of the reaction by activating the halogen source and stabilizing the resulting radical intermediates. Another approach involves the use of halogen-donating reagents, such as N-halosuccinimides, which can selectively introduce a halogen atom at the benzylic position without affecting other functional groups in the molecule.
Applications in Drug Discovery and Development:
Allylic and benzylic halogenation reactions have found wide application in the synthesis of new compounds for use in drug discovery and development. By introducing halogen atoms at specific positions in a molecule, these reactions can improve the potency, selectivity, and pharmacokinetic properties of a compound. For example, allylic and benzylic halogenation reactions have been used to synthesize new classes of potential cancer therapeutics and antibiotics.
Conclusion:
Allylic and benzylic halogenation reactions are powerful tools in organic synthesis, allowing for the introduction of halogen atoms at specific positions in a molecule. Recent advancements in the field have improved the efficiency and selectivity of these reactions, opening up new avenues for the synthesis of complex molecules. These reactions have found wide application in drug discovery and development, where they can be used to improve the potency, selectivity, and pharmacokinetic properties of a compound. Further advancements in the field of allylic and benzylic halogenation are expected to lead to the discovery of new compounds with improved therapeutic potential.
