Simple cyclic hydrocarbons are also known as alicyclic hydrocarbons, which are hydrocarbons that contain one or more rings of carbon atoms in their structure. These cyclic hydrocarbons may be classified based on the number of carbon atoms in their ring structure.
Here are some examples of simple cyclic hydrocarbons:
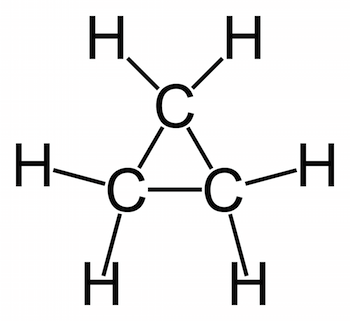
- Cyclopropane: a three-carbon cyclic hydrocarbon with the chemical formula C3H6.
- Cyclobutane: a four-carbon cyclic hydrocarbon with the chemical formula C4H8.
- Cyclopentane: a five-carbon cyclic hydrocarbon with the chemical formula C5H10.
- Cyclohexane: a six-carbon cyclic hydrocarbon with the chemical formula C6H12.
- Cycloheptane: a seven-carbon cyclic hydrocarbon with the chemical formula C7H14.
- Cyclooctane: an eight-carbon cyclic hydrocarbon with the chemical formula C8H16.
These cyclic hydrocarbons are important in organic chemistry and are used in various industrial processes.
What is Required Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
Organic chemistry is the study of carbon-containing compounds, including simple cyclic hydrocarbons. Here are some of the basic principles of organic chemistry:
- Structure of organic molecules: Organic molecules contain carbon atoms that can form covalent bonds with other atoms such as hydrogen, oxygen, nitrogen, and halogens. The structure of an organic molecule determines its physical and chemical properties.
- Functional groups: Organic molecules can contain functional groups, which are specific arrangements of atoms that give the molecule certain properties and reactivity. Common functional groups include alcohols, aldehydes, ketones, carboxylic acids, esters, and amines.
- Isomerism: Isomers are molecules that have the same molecular formula but different structures. Isomerism is an important concept in organic chemistry, as isomers can have different physical and chemical properties.
- Nomenclature: Organic molecules are named using a systematic nomenclature system based on the IUPAC (International Union of Pure and Applied Chemistry) guidelines. The name of an organic molecule reflects its structure and functional groups.
- Chemical reactions: Organic molecules can undergo a variety of chemical reactions, such as oxidation, reduction, substitution, addition, and elimination reactions. These reactions can be used to synthesize new organic molecules or to modify existing ones.
- Stereochemistry: Stereochemistry is the study of the spatial arrangement of atoms in a molecule. This includes the study of stereoisomers, which are molecules that have the same structural formula but different spatial arrangements of atoms.
- Simple cyclic hydrocarbons: Simple cyclic hydrocarbons are important in organic chemistry and are used in various industrial processes. The cyclic nature of these compounds affects their physical and chemical properties, and their reactivity can be modified by the addition of functional groups.
When is Required Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
The basic principles of organic chemistry, including simple cyclic hydrocarbons, are required for understanding and studying a wide range of topics in chemistry and related fields. Here are some examples:
- Biochemistry: Organic chemistry is the foundation of biochemistry, which is the study of the chemical processes that occur in living organisms. Organic molecules such as carbohydrates, proteins, lipids, and nucleic acids are essential components of living cells.
- Materials science: Organic chemistry is important for the development and synthesis of new materials, such as polymers, plastics, and composites.
- Medicinal chemistry: Organic chemistry is crucial for the design and synthesis of new drugs and pharmaceuticals. Medicinal chemists use organic chemistry principles to create compounds that can interact with biological systems and treat diseases.
- Environmental science: Organic chemistry is important for understanding the behavior and fate of organic pollutants in the environment. Environmental scientists use organic chemistry principles to study the sources, transport, and transformation of organic compounds in air, water, and soil.
- Agriculture: Organic chemistry is important in agriculture for the development of pesticides, fertilizers, and other chemicals used in farming.
Overall, a solid understanding of the basic principles of organic chemistry, including simple cyclic hydrocarbons, is essential for anyone interested in pursuing a career or conducting research in chemistry, biochemistry, materials science, medicinal chemistry, environmental science, agriculture, or related fields.
Where is Required Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
The basic principles of organic chemistry, including simple cyclic hydrocarbons, are used and applied in various fields such as:
- Chemical industry: The chemical industry uses organic chemistry principles to synthesize and manufacture a wide range of products, such as plastics, pharmaceuticals, pesticides, and fertilizers.
- Petrochemical industry: Organic chemistry is used in the petrochemical industry for the refining and processing of crude oil into fuels, lubricants, and other products.
- Food industry: Organic chemistry principles are used in the food industry for the development and production of food additives, preservatives, and flavors.
- Environmental science: Organic chemistry principles are used in environmental science for the study of organic pollutants and their effects on the environment.
- Medicine: Organic chemistry is used in medicine for the synthesis and development of drugs and pharmaceuticals.
- Agriculture: Organic chemistry principles are used in agriculture for the development of pesticides, herbicides, and fertilizers.
- Biotechnology: Organic chemistry principles are used in biotechnology for the development and production of biofuels, enzymes, and other bioproducts.
Overall, the principles of organic chemistry, including simple cyclic hydrocarbons, are important and widely applied in many fields where carbon-containing compounds are involved, either in natural or synthetic processes.
How is Required Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
The basic principles of organic chemistry, including simple cyclic hydrocarbons, are studied and applied using a variety of methods and techniques. Here are some examples:
- Spectroscopy: Spectroscopic methods, such as infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry, are used to analyze the structure and properties of organic molecules. Spectroscopy allows chemists to identify functional groups, determine the connectivity of atoms in a molecule, and study molecular vibrations and electronic transitions.
- Synthesis: Organic chemists use various synthesis methods to create new organic molecules or modify existing ones. Synthesis methods include reactions such as substitution, addition, elimination, and oxidation/reduction.
- Chromatography: Chromatographic methods, such as gas chromatography (GC) and liquid chromatography (LC), are used to separate and analyze complex mixtures of organic molecules. Chromatography allows chemists to isolate and purify specific compounds from a mixture.
- Computational methods: Computational methods, such as molecular modeling and quantum chemistry, are used to study the structure, properties, and reactivity of organic molecules. Computational methods allow chemists to predict and optimize the behavior of organic molecules in various chemical reactions.
- X-ray crystallography: X-ray crystallography is a powerful technique used to determine the three-dimensional structure of organic molecules at the atomic level. X-ray crystallography provides detailed information about the spatial arrangement of atoms in a molecule, which is essential for understanding the molecule’s properties and behavior.
Overall, a combination of these and other methods and techniques are used to study and apply the basic principles of organic chemistry, including simple cyclic hydrocarbons. These methods and techniques enable researchers to develop new organic compounds, optimize chemical reactions, and understand the behavior of organic molecules in various environments and applications.
Production of Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
Basic principles of organic chemistry, including simple cyclic hydrocarbons, are not produced, but they are studied and applied to various chemical processes, reactions, and applications. However, the organic compounds themselves can be produced using various methods and techniques, depending on their structures and properties. Here are some examples:
- Synthesis: Organic compounds, including simple cyclic hydrocarbons, can be synthesized using various chemical reactions, such as addition, elimination, substitution, oxidation, and reduction. The choice of reaction depends on the starting materials and desired products.
- Fermentation: Organic compounds, such as ethanol and lactic acid, can be produced through fermentation processes using microorganisms such as bacteria or yeasts.
- Extraction: Organic compounds, such as essential oils and natural dyes, can be extracted from plant or animal sources using various extraction methods, such as steam distillation, solvent extraction, or supercritical fluid extraction.
- Biotechnology: Organic compounds, such as biofuels, enzymes, and amino acids, can be produced using biotechnological methods, such as genetic engineering, fermentation, or enzymatic catalysis.
- Petrochemical processes: Organic compounds, such as benzene and cyclohexane, can be produced from petroleum and other fossil fuels through various petrochemical processes, such as cracking and reforming.
Overall, the production of organic compounds, including simple cyclic hydrocarbons, requires a thorough understanding of organic chemistry principles and the use of various methods and techniques, depending on the desired product and application.
Case Study on Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
Case Study: The Synthesis of Cyclohexene from Cyclohexanol
Cyclohexene is a simple cyclic hydrocarbon that is widely used as a solvent, a starting material for the synthesis of other compounds, and as a monomer in the production of plastics. It can be synthesized through the dehydration of cyclohexanol using an acid catalyst.
The basic principles of organic chemistry, such as the mechanisms of acid-catalyzed dehydration and the properties of alcohols and alkenes, are essential to understanding and optimizing the synthesis of cyclohexene. Here is an overview of the synthesis process:
- Starting Material: Cyclohexanol is the starting material for the synthesis of cyclohexene. Cyclohexanol is a secondary alcohol that contains a hydroxyl (-OH) group attached to a carbon atom that is bonded to two other carbon atoms in a cyclic six-membered ring.
- Acid-catalyzed Dehydration: Cyclohexanol is dehydrated to cyclohexene by removing a water molecule (-H2O) from the hydroxyl group. The reaction is catalyzed by an acid, such as sulfuric acid (H2SO4), which protonates the hydroxyl group, making it more reactive towards elimination.
- Mechanism of Dehydration: The mechanism of acid-catalyzed dehydration involves the formation of a carbocation intermediate, followed by the loss of a proton from a neighboring carbon atom to form the alkene product. The mechanism is known as an E1 reaction, which involves a unimolecular elimination step.
- Product Formation: The final product of the synthesis is cyclohexene, which is an unsaturated cyclic hydrocarbon with a double bond between two carbon atoms in the six-membered ring.
Overall, the synthesis of cyclohexene from cyclohexanol demonstrates the basic principles of organic chemistry, including the mechanisms of acid-catalyzed dehydration and the properties of alcohols and alkenes. The synthesis process also highlights the importance of understanding the structure and properties of organic compounds and the use of appropriate reaction conditions and catalysts to optimize the yield and purity of the product.
White paper on Basic Principles of Organic Chemistry Including simple cyclic hydrocarbons
Introduction:
Organic chemistry is the study of carbon-containing compounds, including simple cyclic hydrocarbons. The basic principles of organic chemistry are essential to understanding the structure, properties, and reactions of these compounds. This white paper aims to provide an overview of the basic principles of organic chemistry, with a focus on simple cyclic hydrocarbons.
Basic Principles of Organic Chemistry:
- Structure of Organic Compounds: Organic compounds are composed of carbon atoms that can form covalent bonds with other carbon atoms, hydrogen, oxygen, nitrogen, and other elements. The arrangement of atoms in a molecule determines the physical and chemical properties of the compound.
- Bonding and Hybridization: Carbon atoms in organic compounds can form single, double, or triple covalent bonds with other atoms. The bonding is influenced by the hybridization of the carbon atom, which determines the geometry and stability of the molecule.
- Functional Groups: Organic compounds contain functional groups, which are specific arrangements of atoms that give the molecule its characteristic properties and reactivity. Examples of functional groups in cyclic hydrocarbons include alkene, alkyne, and cycloalkane.
- Isomerism: Organic compounds can exist in different forms called isomers, which have the same molecular formula but different structures and properties. Isomerism is especially prevalent in cyclic hydrocarbons, where different arrangements of carbon atoms in the ring can lead to isomers with different properties.
- Reactivity: Organic compounds can undergo various reactions, including addition, elimination, substitution, oxidation, and reduction. The reactivity of a compound is influenced by its structure, functional groups, and the reaction conditions.
Synthesis of Simple Cyclic Hydrocarbons:
The synthesis of simple cyclic hydrocarbons, such as cyclohexene, involves various methods, such as acid-catalyzed dehydration of alcohols, cycloaddition reactions, and Diels-Alder reactions. The choice of synthesis method depends on the starting material and desired product.
Applications of Simple Cyclic Hydrocarbons:
Simple cyclic hydrocarbons are widely used as solvents, monomers in polymer production, and starting materials for the synthesis of other compounds. They also have applications in the food, fragrance, and pharmaceutical industries.
Conclusion:
The basic principles of organic chemistry, including the structure, bonding, functional groups, isomerism, and reactivity of organic compounds, are essential to understanding the properties and reactions of simple cyclic hydrocarbons. The synthesis and applications of cyclic hydrocarbons demonstrate the importance of organic chemistry principles in various industries and fields. A deeper understanding of organic chemistry principles can lead to the development of new and innovative applications of simple cyclic hydrocarbons.