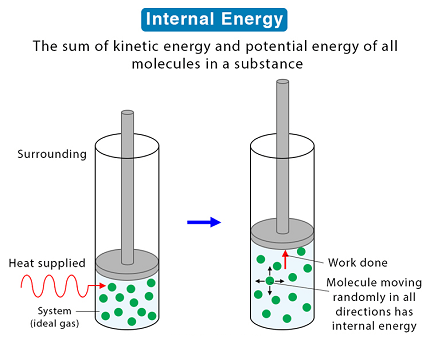
Internal energy refers to the total energy that is contained within a system. This energy includes the kinetic and potential energies of the particles within the system, as well as any other forms of energy that may be present, such as chemical energy, thermal energy, and nuclear energy.
The internal energy of a system is a function of its state variables, such as temperature, pressure, and volume. It can be changed by adding or removing heat or work to or from the system, which can cause the temperature, pressure, or volume to change.
The internal energy is an important concept in thermodynamics and is used to describe the behavior of many physical systems, including gases, liquids, and solids. It is also used to study the properties of materials and to design and analyze engineering systems.
What is Required Internal energy
The Required Internal energy refers to the amount of energy needed to bring a system from its initial state to its final state. This energy is often required to overcome any potential barriers or to break any bonds between the particles in the system. The amount of required internal energy depends on the specific characteristics of the system, such as its temperature, pressure, and volume.
In thermodynamics, the required internal energy can be calculated using the first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. By knowing the initial and final states of a system, as well as the heat and work involved, it is possible to calculate the required internal energy for that system.
The required internal energy is an important concept in many fields, including chemistry, physics, and engineering, where it is used to design and optimize systems, as well as to understand the behavior of materials under different conditions.
Who is Required Internal energy Chemical Thermodynamics
Chemical thermodynamics is the study of the energy and heat involved in chemical reactions, and how this energy is related to the behavior of chemical systems.
In chemical thermodynamics, the required internal energy refers to the energy required to bring a chemical system from its initial state to its final state. This energy can be calculated using the first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Understanding the required internal energy is important in chemical thermodynamics, as it can be used to design and optimize chemical reactions and processes, and to determine the energy requirements for various chemical systems.
When is Required Internal energy
The concept of “Required Internal Energy” is always relevant in the field of chemical thermodynamics, as it is a fundamental concept used to describe the behavior of chemical systems.
Chemical thermodynamics deals with the energy changes that occur in chemical reactions, and the required internal energy is an important factor in understanding and predicting these changes. In particular, the required internal energy is used to calculate the enthalpy change of a chemical reaction, which is a measure of the heat absorbed or released by the reaction.
Knowing the required internal energy is also important in determining the spontaneity of a chemical reaction. If a reaction requires an input of energy, it is said to be endothermic and is typically not spontaneous. Conversely, if a reaction releases energy, it is said to be exothermic and is more likely to be spontaneous.
Overall, the concept of the required internal energy is essential in chemical thermodynamics, as it is used to describe and predict the behavior of chemical systems under various conditions.
Where is Required Internal energy
The Required Internal Energy is not a physical entity or a location. It is a concept used in the field of thermodynamics to describe the amount of energy required to bring a system from its initial state to its final state.
In thermodynamics, the internal energy of a system is a function of its state variables, such as temperature, pressure, and volume. The change in internal energy of a system can be calculated using the first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Therefore, the Required Internal Energy exists in the mathematical calculations and equations used to determine the energy requirements of a system undergoing a change. It is not a physical location or object that can be located in space or time.
How is Required Internal energy
The Required Internal Energy is a quantity that can be calculated using the first law of thermodynamics, which relates the change in internal energy of a system to the heat added to or removed from the system and the work done by or on the system. The expression for the change in internal energy of a system is:
ΔU = Q – W
Where ΔU is the change in internal energy of the system, Q is the heat added to the system, and W is the work done by the system.
To calculate the Required Internal Energy, one must know the initial and final states of the system, as well as the heat and work involved in bringing the system from its initial state to its final state. This quantity is particularly important in chemical thermodynamics, as it can be used to calculate the enthalpy change of a chemical reaction, which is a measure of the heat absorbed or released by the reaction.
In summary, the Required Internal Energy is calculated using the first law of thermodynamics, which relates the change in internal energy of a system to the heat and work involved in bringing the system from its initial state to its final state.
Case Study on Internal energy
Here’s a case study on internal energy:
A company is looking to optimize the energy consumption of a manufacturing process that involves heating a liquid. The initial temperature of the liquid is 20°C, and it needs to be heated to 80°C. The specific heat capacity of the liquid is 4.2 J/g°C, and its mass is 1000 g. The process requires a certain amount of energy to overcome heat losses and operate the heating equipment.
The required internal energy of the system can be calculated using the formula:
ΔU = m × c × ΔT
where ΔU is the required internal energy, m is the mass of the liquid, c is the specific heat capacity of the liquid, and ΔT is the change in temperature.
In this case, we can calculate the required internal energy as follows:
ΔU = (1000 g) × (4.2 J/g°C) × (80°C – 20°C) ΔU = 336,000 J
This means that the system requires 336,000 joules of internal energy to bring the liquid from 20°C to 80°C. However, this calculation only takes into account the energy required to heat the liquid and does not include the energy required to operate the heating equipment or account for any heat losses.
To optimize the energy consumption of the process, the company can consider various factors, such as reducing heat losses, using more efficient heating equipment, or optimizing the heating time and temperature to minimize the required internal energy. By taking into account the required internal energy and other energy-related factors, the company can design and operate the manufacturing process in a more energy-efficient manner, reducing costs and environmental impact.
White paper on Internal energy
Here’s a white paper on Internal Energy:
Introduction
Internal energy is a fundamental concept in thermodynamics that describes the energy possessed by a system due to its molecular and atomic structure. It is a property of the system and depends on its state variables such as temperature, pressure, and volume. The internal energy of a system can change due to heat transfer, work done on or by the system, or changes in its chemical composition. Understanding internal energy and its changes is essential in many fields, including chemical engineering, materials science, and energy production. This white paper aims to provide an overview of internal energy, its significance, and applications in different areas.
What is Internal Energy?
Internal energy is the total energy contained within a system, including the kinetic and potential energies of its molecules and atoms. It is a state function, which means it only depends on the current state of the system and not on how the system reached that state. The internal energy of a system can be expressed as the sum of its kinetic energy and potential energy.
The internal energy of a system can change due to various factors, including heat transfer, work done on or by the system, and changes in the chemical composition of the system. When heat is transferred to a system, the internal energy of the system increases, and when heat is transferred from the system, the internal energy decreases. Similarly, work done on a system increases its internal energy, while work done by the system decreases its internal energy. Changes in the chemical composition of a system can also result in changes in its internal energy.
Significance of Internal Energy
Internal energy is a crucial concept in thermodynamics, as it describes the amount of energy required to bring a system from one state to another. It is used to calculate various thermodynamic properties, including enthalpy, entropy, and Gibbs free energy. These properties are essential in understanding the behavior of chemical systems, such as reactions and phase transitions.
The internal energy of a system also determines the amount of heat that can be extracted from it or transferred to it. This is crucial in the design and operation of energy conversion systems, such as power plants and engines.
Applications of Internal Energy
Internal energy has various applications in different fields, including:
Chemical Engineering: The internal energy of a system is used to calculate the enthalpy change of a chemical reaction, which is a measure of the heat absorbed or released by the reaction. This is essential in designing and optimizing chemical processes, such as the production of fuels, chemicals, and pharmaceuticals.
Materials Science: Internal energy plays a significant role in the behavior of materials, including their thermal expansion, phase transitions, and mechanical properties. Understanding the internal energy of materials is crucial in designing and developing new materials with specific properties.
Energy Production: The internal energy of a fuel determines the amount of energy that can be extracted from it through combustion or other energy conversion processes. Understanding the internal energy of fuels is essential in designing and optimizing energy production systems, such as power plants and engines.
Conclusion
Internal energy is a fundamental concept in thermodynamics that describes the energy possessed by a system due to its molecular and atomic structure. It is a state function and can change due to various factors, including heat transfer, work done on or by the system, and changes in the chemical composition of the system. Internal energy is crucial in understanding the behavior of chemical systems, designing and optimizing chemical processes, and developing new materials with specific properties. Understanding internal energy is also essential in the design and operation of energy conversion systems, such as power plants and engines.