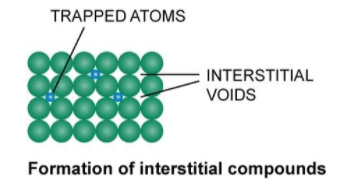
Interstitial compounds are compounds that are formed by inserting atoms or smaller molecules into the interstices or voids of a host crystal lattice. These compounds are usually formed by chemically reacting the host lattice with an appropriate guest molecule or atom.
One example of an interstitial compound is interstitial solid solutions, which are formed when atoms of a foreign element are inserted into the interstices of a host crystal lattice. Another example is interstitial oxides, which are formed when oxygen atoms occupy interstitial sites in a metal lattice.
Interstitial compounds can have unique physical and chemical properties that differ from those of the host lattice or the guest molecule alone. For example, interstitial solid solutions can have improved mechanical properties, such as increased hardness and strength, while interstitial oxides can have high ionic conductivity and catalytic activity.
Interstitial compounds are important in a variety of fields, including materials science, chemistry, and physics. They have applications in catalysis, energy storage, electronic and optical devices, and many other areas.
What is Required Interstitial compounds
Required Interstitial compounds are compounds that are necessary for certain chemical reactions or physical processes to occur. They are typically formed by inserting smaller atoms or molecules into the interstitial sites of a host lattice, creating a new compound with unique properties.
For example, in the process of producing steel, carbon atoms are required to be inserted into the interstitial sites of the iron lattice. The resulting compound, known as interstitial steel, has improved strength and hardness compared to pure iron.
Another example of a required interstitial compound is interstitial hydrides, which are formed when hydrogen atoms occupy the interstitial sites in a metal lattice. These compounds have high hydrogen storage capacity and are being studied for use in fuel cells and other energy storage applications.
In general, required interstitial compounds have important applications in materials science, chemistry, and physics, as they can be used to create new materials with improved properties or enable new chemical reactions to occur.
When is Required Interstitial compounds
Required interstitial compounds are needed when certain properties or functionalities are required that cannot be achieved with the pure host lattice or guest molecules alone. They are typically formed by inserting smaller atoms or molecules into the interstitial sites of a host lattice, creating a new compound with unique properties.
In many cases, required interstitial compounds are used to enhance the mechanical or chemical properties of materials. For example, interstitial alloys can be created by inserting small atoms of another element into the lattice of a metal, resulting in a material with improved strength, hardness, and corrosion resistance.
In some cases, required interstitial compounds are used to enable new chemical reactions to occur. For example, interstitial hydrides, such as palladium hydride, can be used as a catalyst in a variety of chemical reactions.
Required interstitial compounds can also be important in energy storage and conversion technologies. For example, interstitial hydrides have been studied for use in hydrogen storage for fuel cells and batteries, and interstitial compounds such as metal-organic frameworks (MOFs) have been investigated for use in gas storage and separation applications.
Overall, required interstitial compounds are used in a wide range of applications where specific properties or functionalities are required that cannot be achieved with the pure host lattice or guest molecules alone.
Where is Required Interstitial compounds
Required interstitial compounds can be found in a wide range of materials and applications. They are typically formed by inserting smaller atoms or molecules into the interstitial sites of a host lattice, creating a new compound with unique properties.
One example of where required interstitial compounds can be found is in the field of materials science. In this field, interstitial compounds are used to enhance the mechanical or chemical properties of materials. For example, interstitial alloys can be created by inserting small atoms of another element into the lattice of a metal, resulting in a material with improved strength, hardness, and corrosion resistance.
Required interstitial compounds can also be found in the field of catalysis. In this field, interstitial compounds are used as catalysts to enable new chemical reactions to occur. For example, interstitial hydrides, such as palladium hydride, can be used as a catalyst in a variety of chemical reactions.
In the field of energy storage and conversion, required interstitial compounds are being studied for use in hydrogen storage for fuel cells and batteries, and interstitial compounds such as metal-organic frameworks (MOFs) have been investigated for use in gas storage and separation applications.
Overall, required interstitial compounds can be found in a wide range of fields and applications where specific properties or functionalities are required that cannot be achieved with the pure host lattice or guest molecules alone.
How is Required Interstitial compounds
Required interstitial compounds are typically formed by inserting smaller atoms or molecules into the interstitial sites of a host lattice, creating a new compound with unique properties. The process of forming required interstitial compounds can vary depending on the specific materials and application, but some common methods include:
- Diffusion: This method involves heating a mixture of the host lattice and guest atoms or molecules to a high temperature, allowing the guest atoms or molecules to diffuse into the interstitial sites of the host lattice.
- Electrodeposition: This method involves applying an electric current to a solution containing the host lattice and guest atoms or molecules, causing the guest atoms or molecules to be deposited onto the surface of the host lattice and diffuse into the interstitial sites.
- Ion implantation: This method involves bombarding the host lattice with high-energy ions of the guest atoms or molecules, causing them to penetrate the surface of the host lattice and occupy interstitial sites.
- Chemical reaction: This method involves chemically reacting the host lattice with a compound that contains the guest atoms or molecules, resulting in the formation of the required interstitial compound.
The method chosen will depend on the specific application, the materials being used, and the desired properties of the resulting compound. Once formed, required interstitial compounds can have unique physical and chemical properties that differ from those of the host lattice or the guest molecule alone.
Production of Interstitial compounds
Interstitial compounds can be produced using a variety of methods, depending on the specific materials involved and the desired properties of the resulting compound. Some common methods for producing interstitial compounds include:
- Alloying: This method involves mixing two or more metals together to form an alloy. In many cases, the alloy will contain interstitial compounds where smaller atoms of one metal are inserted into the interstitial sites of the other metal.
- Chemical vapor deposition: This method involves heating a substrate and exposing it to a gas mixture containing the desired elements. The gas molecules will react with the substrate to deposit a thin film of interstitial compound onto the substrate.
- Ion implantation: This method involves using a high-energy ion beam to insert atoms of the desired element into the interstitial sites of the host material. This process is often used in semiconductor manufacturing to introduce dopant atoms into a crystal lattice.
- Electrodeposition: This method involves applying an electric current to a solution containing the desired elements, causing them to be deposited onto the surface of a substrate and diffuse into the interstitial sites.
- Diffusion: This method involves heating a mixture of the host material and the desired element to a high temperature, allowing the element to diffuse into the interstitial sites of the host material.
Once the interstitial compound has been produced, it can exhibit unique physical and chemical properties that differ from those of the host material or the guest element alone. These properties can be used in a variety of applications, such as in the production of new materials with enhanced mechanical or chemical properties, or in the development of new catalysts for chemical reactions.
Case Study on Interstitial compounds
One example of the use of interstitial compounds is in the production of high-strength steel for use in automotive and aerospace applications. High-strength steels are alloys that contain small amounts of interstitial elements, such as carbon or nitrogen, that are inserted into the interstitial sites of the steel lattice.
The interstitial elements introduce lattice strain, which increases the strength and hardness of the steel. The amount and type of interstitial elements added to the steel can be carefully controlled to achieve the desired properties.
For example, high-strength low-alloy (HSLA) steels contain small amounts of carbon and other interstitial elements, which make them stronger and more durable than traditional steels. HSLA steels are used in the construction of bridges, buildings, and other structures, as well as in automotive applications.
Another example of the use of interstitial compounds is in the production of interstitial hydrides, which can be used as catalysts for a variety of chemical reactions. Interstitial hydrides are formed by inserting hydrogen atoms into the interstitial sites of metals such as palladium, platinum, or titanium.
The hydrogen atoms can react with other molecules or atoms to facilitate chemical reactions, making interstitial hydrides useful in a variety of catalytic applications, including hydrogenation reactions, dehydrogenation reactions, and fuel cell applications.
Overall, interstitial compounds are a powerful tool in materials science and catalysis, allowing for the creation of new materials with enhanced properties and the development of new catalysts for chemical reactions.
White paper on Interstitial compounds
Introduction:
Interstitial compounds are materials in which atoms or small molecules are inserted into the interstitial sites of a crystal lattice, creating a new compound with unique properties. The inserted atoms or molecules are typically smaller than the host atoms, and their insertion can create lattice strain that alters the physical and chemical properties of the compound. Interstitial compounds have been used in a variety of applications, including in the production of high-strength steels, interstitial hydrides, and catalysts for chemical reactions.
Properties of Interstitial Compounds:
The insertion of interstitial atoms or molecules into a crystal lattice can alter the physical and chemical properties of the resulting compound. For example, the insertion of carbon atoms into the interstitial sites of iron can create high-strength steels with improved mechanical properties, such as increased hardness, tensile strength, and wear resistance. Interstitial compounds can also exhibit unique electronic, optical, and magnetic properties that differ from those of the host material or the guest atom or molecule alone.
Production of Interstitial Compounds:
Interstitial compounds can be produced using a variety of methods, including alloying, chemical vapor deposition, ion implantation, electrodeposition, and diffusion. The method chosen will depend on the specific materials involved and the desired properties of the resulting compound.
Applications of Interstitial Compounds:
Interstitial compounds have a wide range of applications in materials science and catalysis. High-strength steels are used in automotive and aerospace applications due to their improved mechanical properties. Interstitial hydrides are used as catalysts for a variety of chemical reactions, including hydrogenation and dehydrogenation reactions. Other applications of interstitial compounds include the development of new materials with enhanced properties, such as improved electrical conductivity, as well as in the production of fuel cells and other energy technologies.
Conclusion:
Interstitial compounds have revolutionized materials science and catalysis by providing a way to create new compounds with unique properties that cannot be achieved by other means. The use of interstitial compounds has led to the development of high-strength steels, interstitial hydrides, and other materials with enhanced properties, and has opened up new avenues for research in materials science and catalysis.