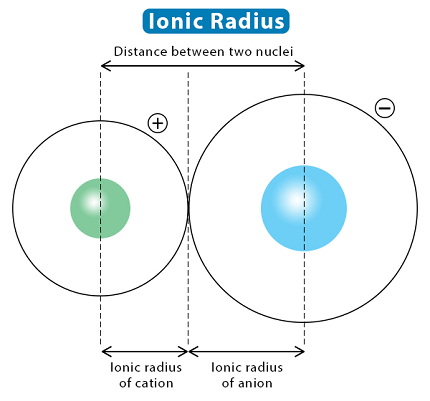
Ionic radius refers to the size of an ion, which is an atom or a group of atoms that has gained or lost one or more electrons, resulting in a net positive or negative charge, respectively.
The ionic radius is defined as half the distance between the nuclei of two ions that are just touching each other when they are in a solid crystal lattice. The size of an ion depends on several factors, including the number of protons and electrons, the electronic configuration, and the type of ion.
In general, cations (positively charged ions) are smaller than their corresponding neutral atoms because they have lost one or more electrons, resulting in a decrease in electron-electron repulsion and a smaller electron cloud. Anions (negatively charged ions) are larger than their corresponding neutral atoms because they have gained one or more electrons, resulting in an increase in electron-electron repulsion and a larger electron cloud.
The ionic radius can be measured experimentally using X-ray crystallography or other techniques, and it is usually reported in picometers (pm) or angstroms (Å). The ionic radius is an important property of ions because it affects their chemical and physical behavior, including their reactivity, solubility, and ability to form chemical bonds.
What is Required Ionic radius
Required ionic radius is the size of an ion that can fit into a particular crystal structure or lattice, without causing significant distortion or disruption to the surrounding atoms or ions.
In many cases, the crystal structure of a material is determined by the size and charge of the ions that make up the material. For example, in a crystal of sodium chloride (NaCl), each sodium ion (Na+) is surrounded by six chloride ions (Cl-) and each chloride ion is surrounded by six sodium ions. The crystal structure of NaCl is determined by the size of the ions and the requirement that each ion is surrounded by ions of the opposite charge.
The required ionic radius can be calculated using the crystal structure of a material and the known sizes of the ions that make up the material. For example, if we know the crystal structure of NaCl and the ionic radii of Na+ and Cl-, we can calculate the required ionic radius for the material.
The required ionic radius is an important concept in materials science and is used to understand and predict the properties of materials, such as their mechanical, electrical, and thermal properties.
When is Required Ionic radius
The concept of required ionic radius is used in materials science and solid-state chemistry when designing or analyzing crystal structures of materials.
For example, when designing a new material, scientists may need to consider the size and charge of the ions that make up the material, and how these ions will arrange themselves in a crystal lattice. The required ionic radius can help determine whether a certain combination of ions can form a stable crystal structure, or whether it will lead to structural defects or instability.
Similarly, when analyzing the properties of a material, scientists may need to consider the size and arrangement of the ions in the crystal lattice. The required ionic radius can help determine how the material will behave under different conditions, such as changes in temperature, pressure, or chemical environment.
In summary, the concept of required ionic radius is used whenever there is a need to understand or predict the behavior of materials based on their crystal structure and the properties of the ions that make up the material.
Where is Required Ionic radius
The concept of required ionic radius is used in solid-state chemistry and materials science, which are fields of study that investigate the properties and behavior of materials, particularly in the solid state.
In these fields, scientists use various techniques to determine the crystal structures of materials and the sizes and charges of the ions that make up the materials. They then use the concept of required ionic radius to understand how these ions will arrange themselves in the crystal lattice and how the crystal structure will affect the properties of the material.
The required ionic radius is an important concept in the design and development of materials with specific properties, such as high strength, conductivity, or magnetism. It is also used in the study of materials under different conditions, such as high temperatures or pressures, or in different chemical environments.
How is Required Ionic radius
The required ionic radius can be calculated using the crystal structure of a material and the known ionic radii of the ions that make up the material.
To calculate the required ionic radius, scientists first determine the crystal structure of the material, which is often done using X-ray diffraction techniques. They then use the crystal structure to determine the coordination number of each ion in the lattice, which is the number of neighboring ions that surround each ion.
Next, they consider the size of the ions and the requirement that each ion is surrounded by ions of the opposite charge in the lattice. The required ionic radius is the size of an ion that can fit into the lattice without causing significant distortion or disruption to the surrounding atoms or ions.
The required ionic radius can be calculated using various models, such as the Pauling’s rules or the Kapustinskii equation, which take into account factors such as the charge, size, and coordination number of the ions.
Experimental techniques such as X-ray crystallography, neutron diffraction, or other spectroscopic methods can also be used to determine the actual size of ions in a crystal lattice, which can be compared to the calculated required ionic radius.
In summary, the required ionic radius can be calculated based on the crystal structure of a material and the properties of the ions that make up the material, and it is an important factor in understanding and predicting the properties of materials.
Production of Ionic radius
Ionic radius is not produced in the traditional sense as it is a physical property that describes the size of an ion. The ionic radius is determined based on experimental measurements and theoretical calculations, and it is a fundamental property of an ion that is related to its electron configuration and charge.
There are different methods used to measure or estimate the ionic radius of an ion, depending on the specific context and the properties of the ion. For example, X-ray diffraction, electron diffraction, and neutron scattering can be used to measure the size of an ion in a crystal structure. Other techniques such as optical spectroscopy, mass spectrometry, and molecular dynamics simulations can also provide information about the ionic radius of ions in various contexts.
In addition, there are different models and theories that can be used to estimate the ionic radius of an ion based on its charge, electron configuration, and other properties. For example, the Shannon’s radii or the Kapustinskii equation are commonly used to estimate the ionic radius of ions based on their coordination number and charge.
In summary, the ionic radius is a physical property of ions that is determined based on experimental measurements or theoretical calculations, and there are different methods and models used to estimate or measure it.
Case Study on Ionic radius
One example of a case study involving the concept of ionic radius is the design and development of solid oxide fuel cells (SOFCs), which are a promising technology for generating electricity from clean fuels such as hydrogen or methane.
SOFCs consist of an electrolyte material, such as yttria-stabilized zirconia (YSZ), sandwiched between two electrodes. The electrodes are made of a porous ceramic material, such as lanthanum strontium manganite (LSM), which acts as the cathode, and nickel or nickel-cermet, which acts as the anode.
The performance of SOFCs depends on the efficiency of the ion transport through the electrolyte material, which is influenced by the size and charge of the ions involved. In particular, the size of the oxygen ions in the YSZ electrolyte is an important factor that affects the ionic conductivity and the overall performance of the SOFC.
To optimize the performance of SOFCs, scientists have studied the relationship between the ionic radius of the oxygen ions in the YSZ electrolyte and the performance of the fuel cell. They have found that the ionic conductivity of YSZ increases as the ionic radius of the oxygen ions decreases, due to the increased electrostatic attraction between the ions and the lattice.
Therefore, researchers have developed methods to reduce the ionic radius of the oxygen ions in YSZ, such as doping the material with elements such as scandium or yttrium. By reducing the ionic radius of the oxygen ions in YSZ, the overall performance of the SOFC can be improved, leading to higher efficiency and lower costs.
In summary, the concept of ionic radius plays an important role in the design and development of materials for various applications, including SOFCs. By understanding and optimizing the ionic radius of the ions involved in a material, scientists can improve its properties and performance, leading to more efficient and effective technologies.
White paper on Ionic radius
Introduction
Ionic radius is a fundamental property of ions that describes their size in a given crystal structure. It is an important parameter that influences many properties of materials, including their chemical reactivity, electrical conductivity, and mechanical strength. In this white paper, we will discuss the concept of ionic radius, its measurement and calculation methods, and its applications in various fields.
Concept of Ionic Radius
Ionic radius refers to the distance between the nucleus and the outermost electron shell of an ion. The size of an ion is affected by several factors, including the number of electrons in the outermost shell, the number of protons in the nucleus, and the charge on the ion. The ionic radius is usually expressed in picometers (pm) or angstroms (Å), and it is determined by measuring the distances between ions in a crystal lattice.
Methods for Measuring Ionic Radius
The ionic radius can be determined using various experimental techniques, such as X-ray diffraction, neutron diffraction, and electron diffraction. In X-ray diffraction, the distance between the atoms in a crystal structure is measured by analyzing the interference pattern of X-rays passing through the crystal. In neutron diffraction, the positions of atoms in a crystal structure are determined by analyzing the diffraction pattern of neutrons. In electron diffraction, the electrons in a crystal structure are scattered by a beam of electrons, and the resulting diffraction pattern is used to determine the distances between atoms.
Calculation of Ionic Radius
The ionic radius can also be estimated using theoretical calculations, such as the Pauling’s rules or the Kapustinskii equation. The Pauling’s rules are a set of empirical rules that relate the ionic radius to the coordination number of the ion and the crystal structure of the material. The Kapustinskii equation is a more advanced model that takes into account the size, charge, and coordination number of the ions in a material.
Applications of Ionic Radius
The concept of ionic radius has many applications in various fields, including materials science, chemistry, and biology. In materials science, the ionic radius is used to design and develop materials with specific properties, such as high ionic conductivity or mechanical strength. In chemistry, the ionic radius is used to predict and understand the reactivity of ions in chemical reactions. In biology, the ionic radius is used to study the properties and functions of ions in biological systems, such as ion channels and transporters.
Conclusion
Ionic radius is a fundamental property of ions that influences many properties of materials and plays an important role in various fields. The measurement and calculation of ionic radius using experimental and theoretical methods are essential for understanding and predicting the properties of materials. The concept of ionic radius has many applications in materials science, chemistry, and biology, and it is an important parameter that affects the behavior of ions in different contexts.
