Ionization enthalpy, also known as ionization energy, is the amount of energy required to remove one electron from an isolated gaseous atom or ion in its ground state. It is a measure of the tendency of an atom or ion to lose an electron.
The first ionization enthalpy is the energy required to remove the first electron from the neutral atom, while the second ionization enthalpy is the energy required to remove the second electron, and so on.
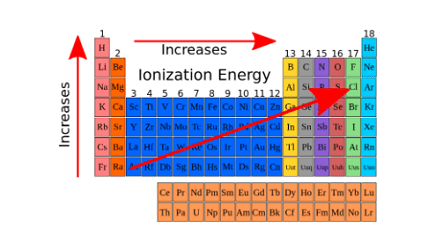
Ionization enthalpy increases as you move from left to right across a period in the periodic table, because the effective nuclear charge increases, making it harder to remove an electron. Ionization enthalpy decreases as you move down a group in the periodic table, because the electrons are further away from the nucleus and therefore experience less attraction to the nucleus.
Ionization enthalpy is an important concept in chemistry, as it helps explain a variety of chemical and physical properties of elements and their compounds.
What is Required Ionization enthalpy
Required ionization enthalpy refers to the minimum amount of energy required to remove an electron from a specific electron shell of an atom or ion.
Each electron shell in an atom or ion has a specific ionization energy, and the required ionization energy increases as the distance from the nucleus increases. This is because the electrons in outer shells experience less attraction to the positively charged nucleus and are therefore more loosely held.
The required ionization energy is also affected by the electronic configuration of the atom or ion. For example, atoms or ions with half-filled or fully filled electron shells have greater stability and require more energy to remove an electron than atoms or ions with partially filled shells.
The concept of required ionization energy is important in understanding chemical reactions and the behavior of elements and their compounds. It can also be used in various applications, such as in ionization energy spectroscopy techniques for identifying and characterizing molecules.
When is Required Ionization enthalpy
“Required Ionization Enthalpy” is not a commonly used term in chemistry, so there is no specific time or context when it is used. However, the term “ionization enthalpy” is used in the context of atomic and molecular physics and chemistry, where it refers to the amount of energy required to remove an electron from an atom or molecule in its ground state.
Ionization enthalpy is a fundamental concept in chemistry and is used to explain various chemical and physical properties of elements and their compounds. It is an important parameter in many chemical reactions and processes, including ionization processes, chemical bonding, and reactivity. It is also used in various spectroscopic techniques for identifying and characterizing molecules.
Where is Required Ionization enthalpy
“Required Ionization Enthalpy” is not a commonly used term in chemistry, so there is no specific physical location or place associated with it.
However, ionization enthalpy, which is the amount of energy required to remove an electron from an isolated gaseous atom or ion in its ground state, can be measured in a laboratory using various experimental techniques. These techniques include photoelectron spectroscopy, where electrons are ejected from a sample using photons, and mass spectrometry, where ions are separated based on their mass-to-charge ratio.
Ionization enthalpy is an important concept in chemistry, and it is used to explain a variety of chemical and physical properties of elements and their compounds. It is also used in many chemical reactions and processes and plays a crucial role in understanding the behavior of elements and their compounds.
How is Required Ionization enthalpy
“Required Ionization Enthalpy” is not a commonly used term in chemistry, so there is no specific method or procedure associated with it.
However, ionization enthalpy, which is the amount of energy required to remove an electron from an isolated gaseous atom or ion in its ground state, can be calculated or measured using various experimental and theoretical methods.
One experimental method for measuring ionization enthalpy is photoelectron spectroscopy, where electrons are ejected from a sample using photons, and the energy required to remove the electron is measured. Another experimental method is mass spectrometry, where ions are separated based on their mass-to-charge ratio.
Theoretical methods for calculating ionization enthalpy include quantum mechanical calculations, which use mathematical models to predict the electronic structure and properties of atoms and molecules.
Ionization enthalpy is an important concept in chemistry, and it is used to explain a variety of chemical and physical properties of elements and their compounds. It is also used in many chemical reactions and processes and plays a crucial role in understanding the behavior of elements and their compounds.
Nomenclature of Ionization enthalpy
The term “ionization enthalpy” is a standard nomenclature used in chemistry to refer to the amount of energy required to remove an electron from an isolated gaseous atom or ion in its ground state. It is also sometimes referred to as ionization energy or ionization potential.
The ionization enthalpy of an atom or ion is typically measured in units of joules (J) or electron volts (eV). The SI unit for ionization enthalpy is the joule, but electron volts are often used in practice because they are more convenient for expressing small energy values.
In addition to the first ionization enthalpy, which is the energy required to remove the first electron from an atom or ion, there are also higher ionization enthalpies, which refer to the energy required to remove subsequent electrons.
The nomenclature for ionization enthalpies can vary depending on the context or the specific application. For example, in spectroscopy, the term “binding energy” may be used to refer to ionization enthalpy. However, in general, the term “ionization enthalpy” is the most commonly used nomenclature in chemistry.
Case Study on Ionization enthalpy
One example of a case study that involves ionization enthalpy is the reactivity of alkali metals. Alkali metals such as lithium, sodium, and potassium are highly reactive due to their low ionization enthalpies.
The first ionization enthalpy of these metals decreases down the group, meaning that it requires less energy to remove an electron from the outermost shell of the atom as you go from lithium to potassium. This makes it easier for alkali metals to lose an electron and form cations, which are positively charged ions.
This low ionization enthalpy makes alkali metals highly reactive with other elements, particularly with halogens such as chlorine and fluorine, which have high electron affinities (the energy change that occurs when an electron is added to a neutral atom). When an alkali metal reacts with a halogen, it loses an electron to form a cation, and the halogen gains an electron to form an anion. The resulting ionic compound is typically a white, crystalline solid with a high melting point.
The reactivity of alkali metals can also be observed in their reaction with water. When alkali metals are added to water, they react vigorously, releasing hydrogen gas and forming an alkaline solution of the metal hydroxide. This reaction is highly exothermic and can even lead to explosions if not carried out carefully.
Overall, the low ionization enthalpy of alkali metals makes them highly reactive with other elements, and this reactivity is responsible for their many industrial and chemical applications, such as in batteries, explosives, and chemical synthesis.
White paper on Ionization enthalpy
Introduction:
Ionization enthalpy is a fundamental concept in chemistry that refers to the amount of energy required to remove an electron from an isolated gaseous atom or ion in its ground state. The ionization enthalpy is an important property of an element, and it is used to explain many chemical and physical properties of elements and their compounds. In this white paper, we will discuss the definition of ionization enthalpy, its importance in chemistry, and some of its applications.
Definition:
Ionization enthalpy is defined as the amount of energy required to remove an electron from an isolated gaseous atom or ion in its ground state. The energy required to remove the first electron is known as the first ionization enthalpy. Subsequent ionization enthalpies are defined as the energy required to remove each additional electron from the ion.
Importance in Chemistry:
Ionization enthalpy is an important property of an element and is used to explain many chemical and physical properties of elements and their compounds. The ionization enthalpy of an element depends on the number of electrons in its outermost shell, known as the valence shell. Elements with low ionization enthalpies tend to be highly reactive, while elements with high ionization enthalpies tend to be less reactive.
The ionization enthalpy of an element is also used to predict the reactivity of that element with other elements. For example, alkali metals such as lithium, sodium, and potassium have low ionization enthalpies and are highly reactive. This reactivity is due to the ease with which they lose electrons to form cations.
Applications:
Ionization enthalpy has many practical applications in chemistry, including in the design and development of new materials and in the study of chemical reactions. The ionization enthalpy of an element can be used to predict the stability of that element in different chemical environments.
Ionization enthalpy is also used in the design of chemical reactions. In chemical synthesis, the ionization enthalpy of an element is used to predict the reactivity of that element with other elements or compounds. This information can be used to design chemical reactions that are more efficient and selective.
Conclusion:
Ionization enthalpy is a fundamental concept in chemistry that is used to explain many chemical and physical properties of elements and their compounds. The ionization enthalpy of an element is an important property that is used to predict the reactivity of that element with other elements and compounds. The applications of ionization enthalpy are vast and include the design and development of new materials and the study of chemical reactions. Understanding ionization enthalpy is crucial for advancing our knowledge of chemistry and for developing new technologies and materials that will benefit society.
