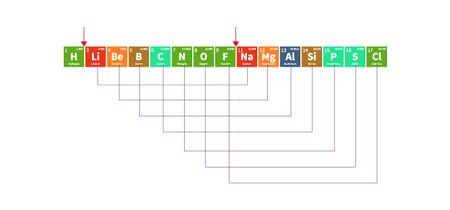
Classification of Elements and Periodicity in Properties is an important topic in the JEE (Main+Advanced) Chemistry syllabus. This topic deals with the arrangement of elements in the periodic table based on their electronic configurations, and the trends in their physical and chemical properties as we move across and down the periodic table.
Some of the key concepts covered in this topic include the electronic configuration of elements, the periodic law and its applications, the periodic trends in atomic and ionic radii, ionization energy, electron affinity, electronegativity, and chemical reactivity. Students are expected to have a good understanding of the periodic table and the periodic trends, and be able to apply this knowledge to solve numerical problems and answer conceptual questions.
To master this topic, students need to have a strong foundation in basic chemistry concepts such as atomic structure, chemical bonding, and chemical reactions. They should also be familiar with the different blocks of the periodic table (s, p, d, and f) and the electronic configurations of elements within each block.
In order to excel in the JEE (Main+Advanced) Chemistry exam, students should focus on developing a deep understanding of the concepts and principles underlying Classification of Elements and Periodicity in Properties, and practice solving a variety of numerical problems and conceptual questions. It is also important to stay updated with the latest developments in this field, and to refer to standard textbooks and reference materials for additional guidance and practice.
What is Required JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
To succeed in the JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties, students need to have a solid foundation in the fundamental concepts of chemistry. Some of the key concepts that are required for this topic include:
- Electronic configuration of elements
- The periodic table and its organization
- Periodic trends in properties such as atomic and ionic radii, ionization energy, electron affinity, electronegativity, and chemical reactivity
- Chemical bonding and the types of bonding that occur between elements
- Chemical reactions and their mechanisms
To excel in this topic, students should focus on developing a deep understanding of the fundamental principles and concepts, and be able to apply them to solve numerical problems and answer conceptual questions. They should also be able to analyze and interpret data related to periodic trends and properties, and understand the implications of these trends in predicting the behavior of elements in different chemical reactions and environments.
Additionally, students should be able to identify and classify elements based on their electronic configurations, and be able to explain the relationship between electronic configuration and the properties of elements. They should also be able to explain the significance of the periodic table and its impact on the organization and classification of elements.
To prepare for the JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties, students should refer to standard textbooks and reference materials, and practice solving a variety of numerical problems and conceptual questions. They should also take mock tests and practice previous years’ question papers to get a feel for the exam format and build their confidence.
Who is Required JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
The JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties is a part of the Chemistry syllabus for students who are preparing for the JEE (Main+Advanced) entrance exam in India. This exam is a competitive exam that is used as the basis for admission to undergraduate engineering programs in prestigious institutions across the country.
Students who wish to pursue a career in engineering or related fields and plan to apply for undergraduate engineering programs in India must take the JEE (Main+Advanced) exam. To be eligible for this exam, students must have completed their 10+2 or equivalent level of education with Physics, Chemistry, and Mathematics as compulsory subjects.
The Classification of Elements and Periodicity in Properties is an important topic in the Chemistry syllabus for the JEE (Main+Advanced) exam. It is required for all students who are appearing for the exam and is given equal weightage as other topics in the Chemistry syllabus.
Therefore, students who wish to appear for the JEE (Main+Advanced) exam and aspire to get admission to undergraduate engineering programs must prepare well for the Classification of Elements and Periodicity in Properties topic in order to score well in the exam and secure a good rank.
When is Required JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
The JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties is a part of the Chemistry syllabus for the JEE (Main+Advanced) entrance exam in India. The JEE (Main) exam is conducted twice a year in the months of January and April, while the JEE (Advanced) exam is conducted once a year in the month of May.
The Classification of Elements and Periodicity in Properties topic is a part of the Chemistry syllabus and is included in both the JEE (Main) and JEE (Advanced) exams. The topic carries significant weightage in the Chemistry section of both exams and is a crucial component of the overall Chemistry syllabus.
Students who are preparing for the JEE (Main+Advanced) exam should allocate sufficient time to prepare for the Classification of Elements and Periodicity in Properties topic. They should focus on developing a deep understanding of the fundamental principles and concepts, and practice solving a variety of numerical problems and conceptual questions to build their proficiency and confidence.
It is important to note that the exact date and time of the exam are subject to change, and students should regularly check the official website of the JEE (Main+Advanced) exam for the latest updates and notifications.
Where is Required JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
The JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties is a part of the Chemistry syllabus for the JEE (Main+Advanced) entrance exam in India. The exam is conducted at various centers across the country, and students can choose their preferred exam center while filling out the application form.
The JEE (Main) exam is conducted in multiple sessions in various cities across India, as well as in some international cities. The JEE (Advanced) exam is typically conducted in major cities across India, with some additional exam centers in other countries.
Students should choose their exam center carefully based on their convenience and accessibility, and ensure that they have sufficient time to reach the exam center on the day of the exam. They should also carry all the necessary documents and materials, such as the admit card, identification proof, and stationery items, to the exam center.
It is important to note that the exact location of the exam center and other relevant details will be mentioned in the admit card, which will be made available to the students before the exam. Students should carefully read and follow all the instructions mentioned in the admit card to avoid any last-minute confusion or inconvenience.
How is Required JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
The JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties is an important topic in the Chemistry syllabus for the JEE (Main+Advanced) entrance exam in India. The topic is aimed at developing the students’ understanding of the periodic trends in the chemical and physical properties of elements and their classification based on their electronic configurations.
To prepare for this topic, students should begin by developing a solid foundation of the fundamental concepts and principles related to the topic. This includes understanding the periodic table, electron configurations, and the periodic trends in properties such as atomic size, ionization energy, electron affinity, electronegativity, and chemical reactivity.
Students can then move on to practicing a variety of numerical problems and conceptual questions to hone their skills and build their confidence. They should also solve previous years’ question papers and mock tests to get a sense of the exam pattern, difficulty level, and types of questions that may be asked in the exam.
In addition to studying the theoretical concepts and solving numerical problems, students should also focus on improving their time management skills and exam-taking strategies. They should attempt to solve the questions in a systematic and organized manner, and manage their time effectively to ensure that they are able to complete the exam within the allotted time.
Overall, the JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties requires a strong understanding of the fundamental concepts, diligent practice, and effective time management skills to perform well in the exam.
Case Study on JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
Case Study:
Rahul is a student preparing for the JEE (Main+Advanced) entrance exam in India. He is currently studying the Classification of Elements and Periodicity in Properties topic in the Chemistry syllabus, which he finds challenging and complex.
To improve his understanding and proficiency in the topic, Rahul adopts a systematic and structured approach to his preparation. He begins by revising the fundamental concepts related to the topic, such as the electronic configurations of elements, periodic table, and periodic trends in properties such as atomic size, ionization energy, electron affinity, and electronegativity.
He then moves on to solving a variety of numerical problems and conceptual questions from various sources, including textbooks, reference books, and online study materials. He also solves previous years’ question papers and mock tests to get a sense of the exam pattern, difficulty level, and types of questions that may be asked in the exam.
As he progresses with his preparation, Rahul realizes that he is struggling with some of the more complex and advanced concepts in the topic, such as the variation of atomic and ionic radii in transition elements, the concept of effective nuclear charge, and the factors affecting ionization energy.
To overcome these challenges, Rahul seeks the help of his teachers and peers, and also enrolls in an online coaching program that specializes in JEE preparation. He attends regular live classes, watches recorded lectures, and interacts with other students and teachers to clarify his doubts and deepen his understanding of the topic.
In addition to his theoretical preparation, Rahul also focuses on improving his time management skills and exam-taking strategies. He attempts to solve the questions in a systematic and organized manner, and manages his time effectively to ensure that he is able to complete the exam within the allotted time.
As a result of his diligent and focused preparation, Rahul is able to perform well in the JEE (Main+Advanced) entrance exam and secure a high rank. He attributes his success to his structured approach, consistent practice, and effective use of study resources and support systems.
Conclusion:
The Classification of Elements and Periodicity in Properties topic in the Chemistry syllabus for the JEE (Main+Advanced) entrance exam in India can be challenging and complex, but with a systematic and structured approach to preparation, students can develop a solid understanding and proficiency in the topic. This includes revising the fundamental concepts, practicing a variety of numerical problems and conceptual questions, seeking help from teachers and peers, and enrolling in coaching programs if necessary. In addition, students should focus on improving their time management skills and exam-taking strategies to perform well in the exam.
White paper on JEE (Main+Advance) e-Intermediate Course Classification of Elements and Periodicity in Properties
Title: Understanding the JEE (Main+Advanced) e-Intermediate Course Classification of Elements and Periodicity in Properties: A White Paper
Introduction:
The JEE (Main+Advanced) entrance exam is one of the most competitive and sought-after exams in India for students seeking admission to prestigious engineering and technology institutes in the country. The Chemistry syllabus for the JEE (Main+Advanced) exam includes the Classification of Elements and Periodicity in Properties topic, which is essential for students to master in order to perform well in the exam.
This white paper aims to provide a comprehensive understanding of the Classification of Elements and Periodicity in Properties topic in the Chemistry syllabus for the JEE (Main+Advanced) entrance exam. It will cover the fundamental concepts, periodic trends, and practical applications of the topic, and provide insights and recommendations for effective preparation and exam-taking strategies.
Fundamental Concepts:
The Classification of Elements and Periodicity in Properties topic is based on the fundamental concepts of electronic configurations and periodic table. Students should have a solid understanding of the electronic configurations of elements, including the concept of valence electrons and their role in chemical bonding.
The periodic table is also an essential component of the topic, and students should be familiar with its layout, including the organization of elements into groups and periods based on their electronic configurations. They should also be aware of the different types of elements, such as metals, non-metals, and metalloids, and their properties.
Periodic Trends:
One of the key aspects of the Classification of Elements and Periodicity in Properties topic is the study of periodic trends in properties of elements, such as atomic size, ionization energy, electron affinity, electronegativity, and chemical reactivity. Students should be aware of how these properties vary across the periodic table and how they are related to the electronic configurations of elements.
For example, atomic size decreases across a period due to increased effective nuclear charge, while it increases down a group due to the addition of new energy levels. Similarly, ionization energy increases across a period due to increased nuclear charge, while it decreases down a group due to increased distance between the nucleus and valence electrons.
Practical Applications:
The Classification of Elements and Periodicity in Properties topic has several practical applications in the fields of chemistry and materials science. For example, the periodic trends in electronegativity can be used to predict the nature of chemical bonding between elements, while the periodic trends in atomic size and ionization energy can be used to predict the reactivity of elements in chemical reactions.
Effective Preparation and Exam-Taking Strategies:
To prepare effectively for the Classification of Elements and Periodicity in Properties topic, students should adopt a structured and systematic approach, which includes revising the fundamental concepts, practicing a variety of numerical problems and conceptual questions, and seeking help from teachers and peers as needed. They should also manage their time effectively during the exam, attempting to solve the questions in a systematic and organized manner.
Conclusion:
In conclusion, the Classification of Elements and Periodicity in Properties topic in the Chemistry syllabus for the JEE (Main+Advanced) entrance exam is a crucial component that students should master in order to perform well in the exam. Students should develop a solid understanding of the fundamental concepts, periodic trends, and practical applications of the topic, and adopt effective preparation and exam-taking strategies to succeed in the exam.
